Compounds accelerating duplication of herpes simplex virus and application
A herpes simplex virus and compound technology, applied in the direction of drug combination, medical preparations containing active ingredients, organic active ingredients, etc., can solve problems such as repeated infection and reinfection, and achieve the effect of promoting virus replication and clear mechanism of action
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034]Example 1 Epigenetic Small Molecular Compounds Promote HSV-1 and HSV-2 Infection Experiment
[0035] (1) Small molecule compound library screening
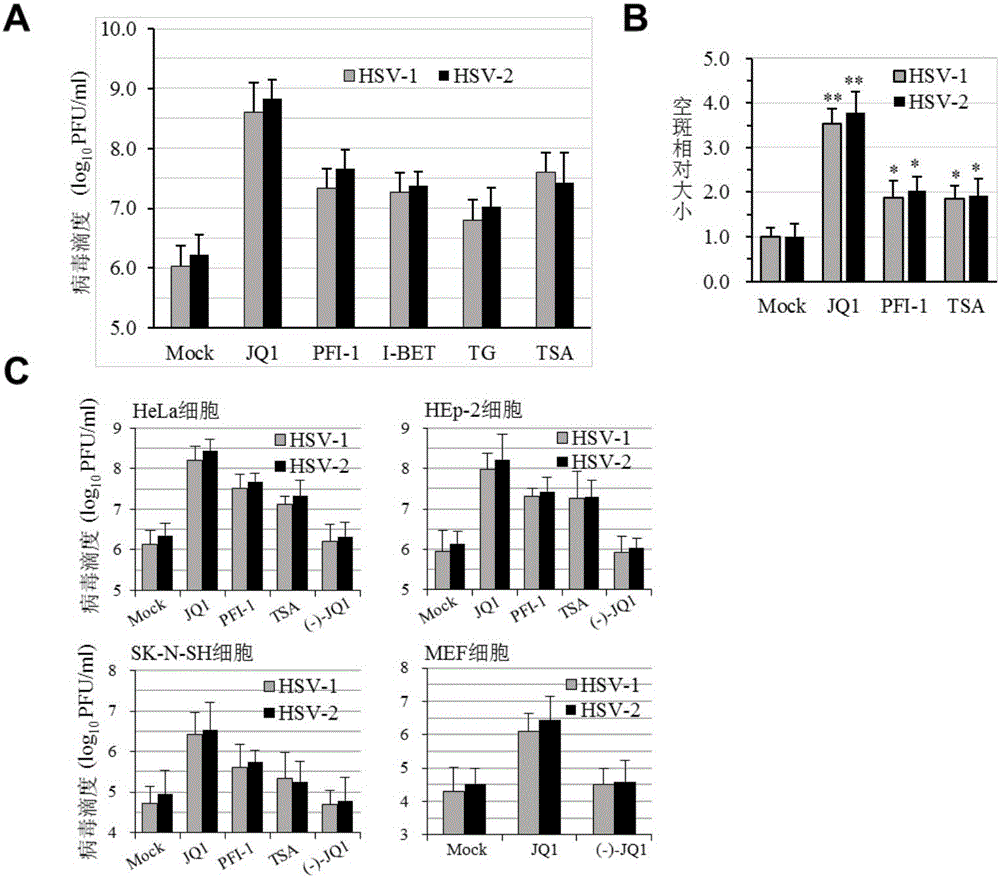
[0036] The day before infection, Vero cells were cultured in 96-well plates, 3x10 per well 3 cell. A small-molecule library containing 129 compounds with less than their cytotoxicity (IC 50 ) 3 times the concentration is the maximum screening concentration (for example, product description marked A compound IC 50 300nM, then the initial maximum concentration is set to 100nM), with DMEM medium as the dilution system, 3-fold dilution to 5 concentrations (take A compound as an example, the test concentration is 100, 33.3, 11.1, 3.7 and 1.2nM ), Vero cells were treated two hours before infection, and the wells were replicated in triplicate. At the same time, a normal control group without drug treatment and a compound toxicity control (using a maximum test concentration of 100 nM as a sample) were set up. Cells were infecte...
Embodiment 2
[0048] Example 2 Determination of Compound JQ-1 Promoting Herpes Simplex Virus Infection Concentration and Time Curve
[0049] (1) Determination of concentration and dosing time
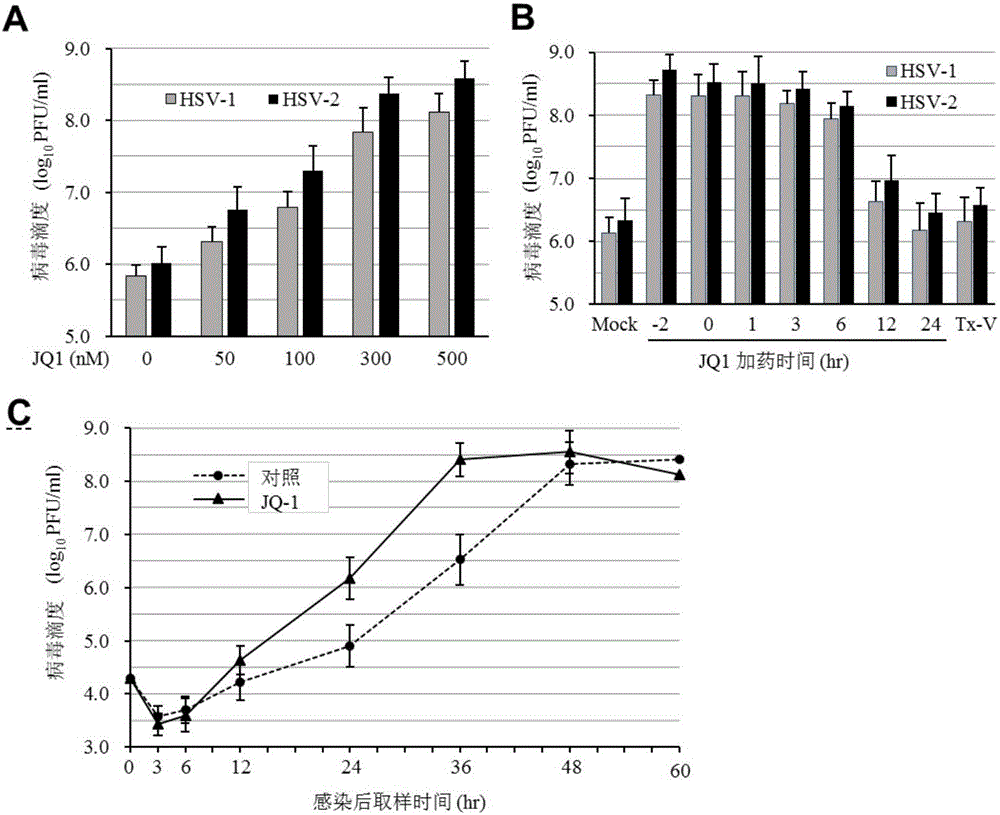
[0050] EC determined according to the above 20 For the data, we selected JQ-1 as a representative, treated Vero cells with 50, 100, 300 and 500nM JQ-1 2 hours before virus infection, and then infected with 1MOI virus in parallel with untreated control. Samples were collected after 36 hours and titrated for virus. The results showed that JQ-1 could promote both HSV-1 and HSV-2 infection at the above concentrations.
[0051] In addition, we also added JQ-1 (300nM) 2 hours before virus infection (-2), at the same time as infection (0), and 1, 3, 6, 12 and 24 hours after virus addition in a similar manner, Cell samples were collected 36 hours after infection, and virus titers were titrated with secondary infections. Adding the compound before infection or in the first 6 hours of infection can signifi...
Embodiment 3
[0054] Example 3 JQ-1 promotes the infection efficiency of HSV-1 (HSV-1 / EGF) carrying the GFP reporter gene.
[0055] HeLa cells were grown overnight in 6-well plates. The cells were treated with JQ-1 (JQ1, 300nM) or (-) JQ-1 without bromodomain inhibitory activity as a control for 2 hours, and then the cells were infected with HSV-1 / GFP (MOI=0.3 and 1.0), and the wells were replicated. Samples were collected 36 hours after infection, the fluorescence intensity of GFP was detected by flow cytometry (FACS), and the mean fluorescence intensity (MFI) was calculated as the effect of JQ-1 on the expression of genes carried by HSV-1. The results showed that JQ-1, but not (-)JQ-1 without bromodomain inhibitory activity, could promote the expression of reporter gene carried by HSV-1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com