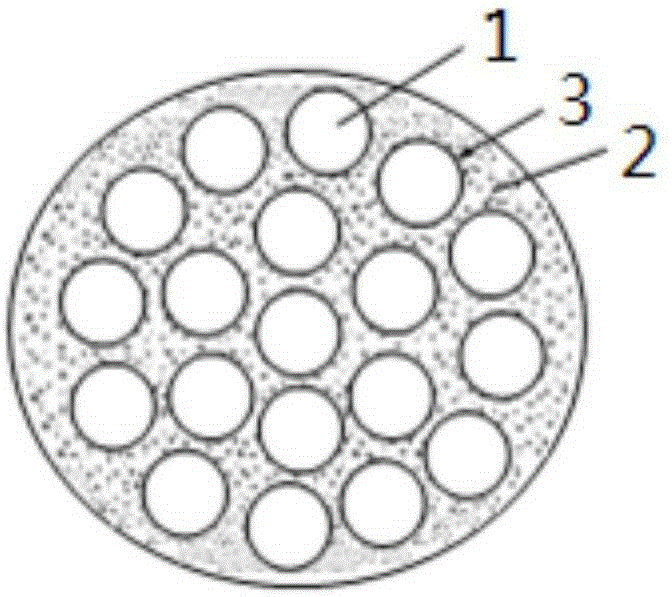
Preparing method for cyclohexane-1,2-dibasic diformate
A technology of dibasic acid dibasic ester and phthalic acid dibasic ester, which is applied in the field of preparation of cyclohexane-1,2-dibasic acid dibasic acid ester, can solve the problem of high energy consumption of hydrogen cycle, low product selectivity, Problems such as large molar ratio of hydrogen ester to achieve the effects of increasing irregularity and dispersion, accelerating reaction rate, and improving selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0035] The invention provides a kind of preparation method of cyclohexane-1,2-dicarboxylate, the method comprises the following steps:
[0036] (1) the hydrogenation raw material containing phthalic acid dibasic ester and H 2performing a first contact in the main hydrogenation reactor in the presence of a first catalyst to obtain a first gas-liquid mixed fluid;
[0037] (2) performing gas-liquid separation on the first gas-liquid mixed fluid;
[0038] (3) injecting hydrogen into the main hydrogenation reaction liquid separated by step (2) through holes with an average pore diameter of nanometer size to obtain a second gas-liquid mixed fluid;
[0039] (4) performing a second contact with the second gas-liquid mixed fluid in the post-hydrogenation reactor in the presence of a second catalyst to obtain a third gas-liquid mixed fluid;
[0040] (5) performing gas-liquid separation on the third gas-liquid mixed fluid.
[0041] According to the present invention, in step (1), the ...
Embodiment 1
[0082] This example is to illustrate the preparation of diisooctyl cyclohexane 1,2-dicarboxylate by the method of the present invention.
[0083] The hydrogenation raw material is the isooctyl alcohol solution and H 2 The first contacting is carried out in the main hydrogenation reactor in the presence of the first catalyst; wherein both the main hydrogenation reactor and the post-hydrogenation reactor are filled with Rh-Sm containing 0.5 wt % Rh, 5.0 wt % Sm / C catalyst; Wherein, the reaction conditions in the main hydrogenation reactor are: reaction temperature 58 ℃, pressure 6.2MPa, H 2 The volume ratio to the hydrogenation raw material is 720, and the liquid space velocity of di-isooctyl phthalate is 0.5h -1 ;
[0084] Results: The conversion rate of diisooctyl phthalate at the outlet of the main hydrogenation reactor was 95.7% by weight, the selectivity of diisooctyl cyclohexane 1,2-dicarboxylate was 99.8%, and the selectivity of cis-cyclohexane 1,2 - The selectivity o...
Embodiment 2
[0089] This example is to illustrate the preparation of diisodecyl cyclohexane 1,2-dicarboxylate by the method of the present invention.
[0090] The raw material for hydrogenation is an isodecyl alcohol solution with a content of 40% by weight of diisodecyl phthalate, and H 2In the presence of the first catalyst, the first contact is carried out in the main hydrogenation reactor; wherein, the Ru-Eu / C catalyst containing 0.5% by weight Ru and 2.0% by weight of Eu is loaded in the main hydrogenation reactor, and after adding Fill the Rh-Yb / Al containing 3.0% by weight Rh, 0.1% by weight Yb in the hydrogen reactor 2 o 3 Catalyst, the reaction conditions in the main hydrogenation reactor are: reaction temperature 40 ℃, pressure 8.0MPa, H 2 The volume ratio to the hydrogenation raw material is 300, and the liquid space velocity of diisodecyl phthalate is 2.0h -1 ;
[0091] Results: The conversion rate of diisodecyl phthalate at the outlet of the main hydrogenation reactor was ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com