Marker as well as preparation method, marking method and applications thereof
A marking method and marker technology, applied in the marker field
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
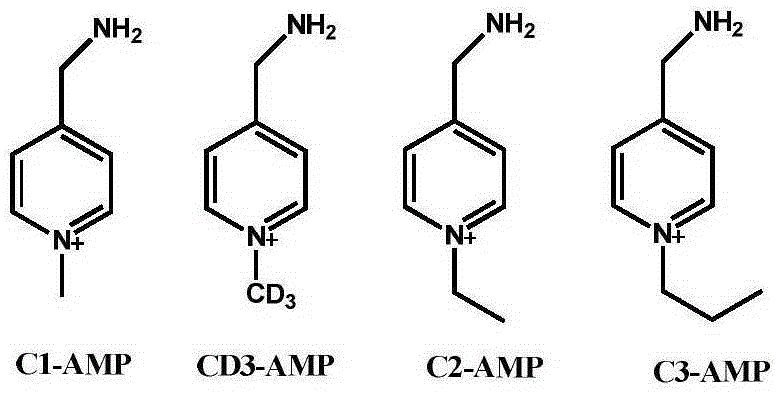
[0059] Embodiment 1 Synthesis of markers of the present invention
[0060] The marker of the present invention is synthesized as follows:
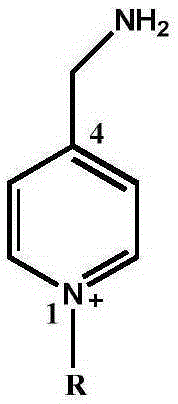
[0061] Mix equimolar free 4-aminomethyl pyridine (4-aminomethylpyridine, abbreviated as: AMP) and phthalic anhydride, then heat to 140-160°C until no water is produced (about 30 minutes), and cool to the residue Compound A was obtained by recrystallization from methanol.
[0062] Dissolve 0.01 mol of compound A in a mixture of 20 mL of methanol and 5 mL of alkyl iodide, put the mixture into a reflux condenser and continue the reaction until the reaction is complete (4-72 hours). The solution was cooled, the solid was filtered off, and compound B was obtained by recrystallization from methanol.
[0063]
[0064] Compound B was put into 20 mL of 48% HBr, and the mixture was put into a reflux condenser to react until a large amount of phthalic acid precipitated. The solution was cooled to room temperature, 20 mL of water was added, and ...
Embodiment 2
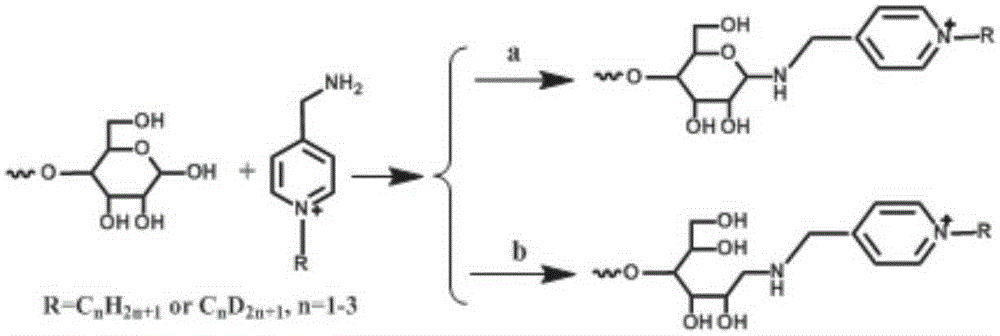
[0067] Embodiment 2 labeling step
[0068] Sugars can be labeled in two different ways, as shown in the following reactions.
[0069]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com