A kind of fused ring aromatic hydrocarbon derivative substituted by phenazine group and application thereof
A condensed-ring aromatic hydrocarbon and phenazine-based technology, applied in the field of organic electroluminescence, can solve the problems of increasing the complexity of the device manufacturing process, reducing the cost of OLEDs, disadvantages, etc., achieving good electron accepting ability, improving luminous efficiency, and reducing working voltage. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
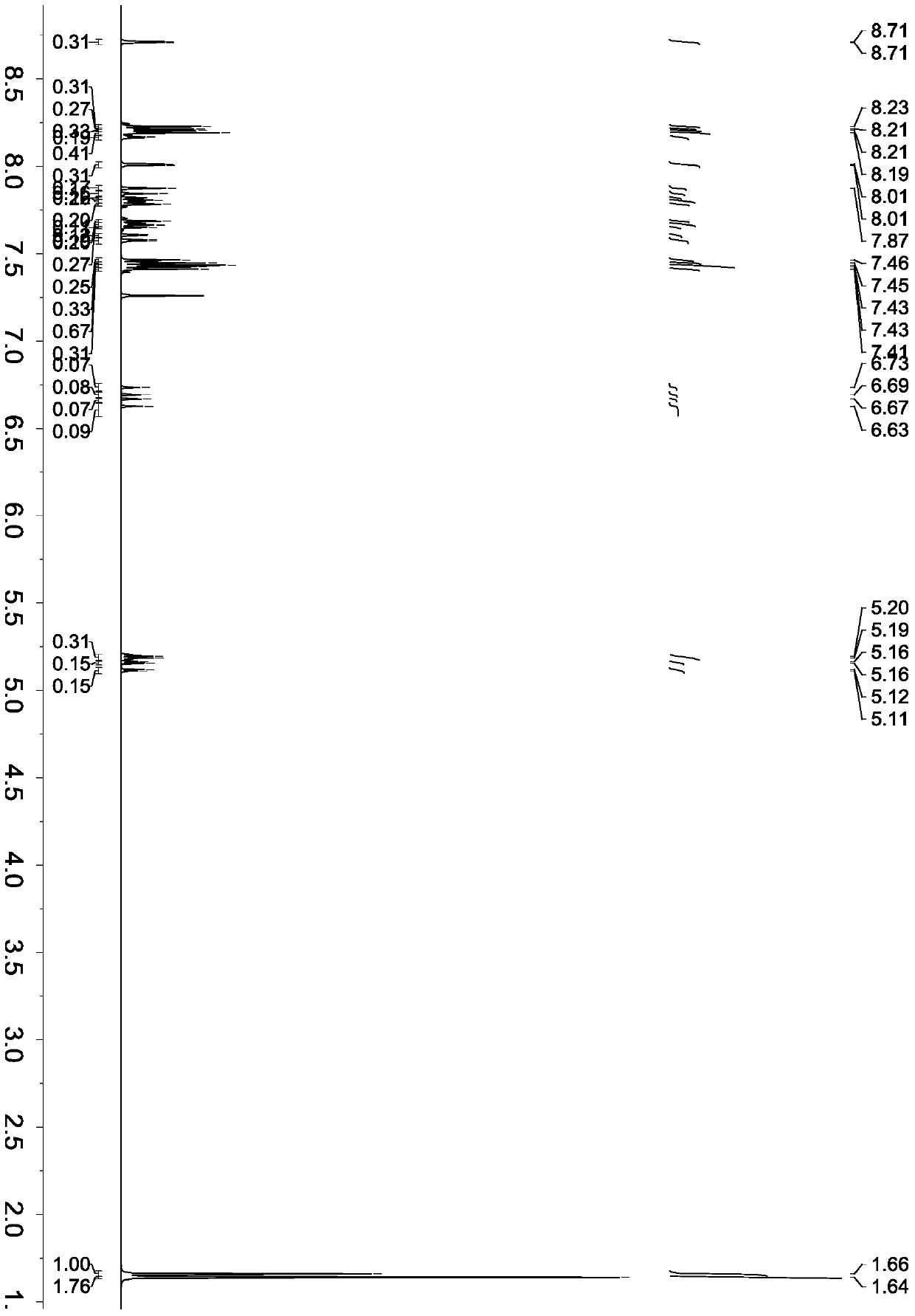
[0064] The synthesis of embodiment 1 2-bromophenazine
[0065]
[0066] Add 3.91 grams of 4-bromo-o-phenylenediamine (molecular weight 186, 0.021 mol), 2.27 grams of o-benzoquinone (molecular weight 108, 0.021 mol), and ethanol (40 ml) into a 250 ml three-necked flask. 0.2 gram of vitriol oil (concentration is 98%), reacted 4 hours at 65 ℃, after reaction finishes, be cooled to room temperature, filter, wash with ethanol, sherwood oil successively, obtain intermediate compound 2-bromophenazine 5.02 grams (molecular weight 258), yield 92.6%.
Embodiment 2
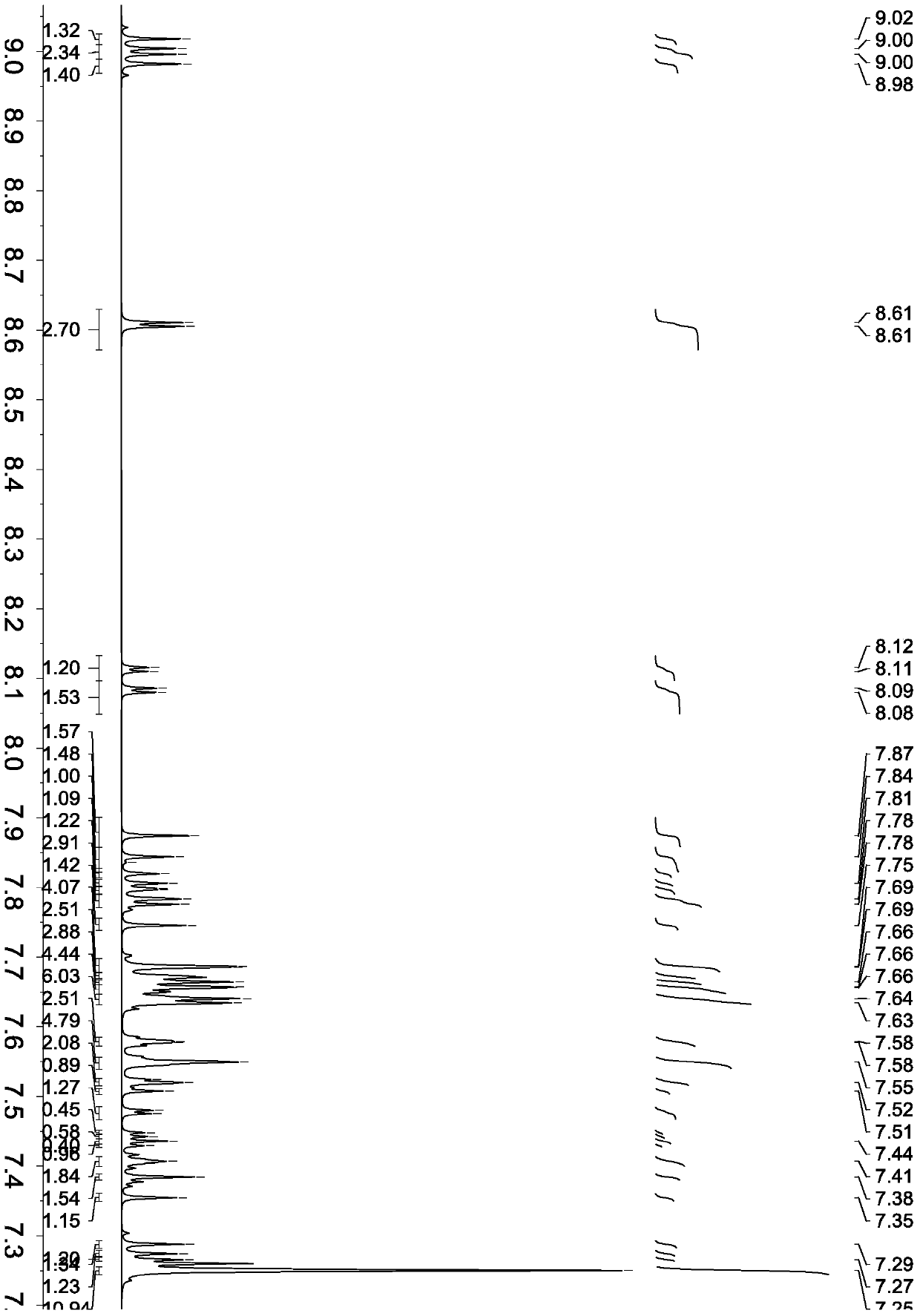
[0068] Synthesis of compound shown in formula (16)
[0069]
[0070] 1000 ml three-necked flask equipped with magnetic stirring and nitrogen protection, add 5.16 g of 2-bromophenazine (molecular weight 258, 0.02 mol), 11.0 g of 9,10-bis(naphthalene-2-yl)anthracene-2-boronic acid (molecular weight 474 , 0.022mol), tetrakis ((triphenylphosphine) palladium 1.16g (molecular weight 1154, 0.001mol), 2M sodium carbonate aqueous solution 80ml, toluene 80ml, ethanol 80ml. After argon replacement, reflux, use thin-layer chromatography ( TLC) method monitoring reaction, after 4.5 hours, TLC finds that raw material bromide reaction is complete, only product point.Lowering temperature, separates organic layer, evaporates to dryness, column chromatography, ethyl acetate / petroleum ether washing, obtains 9.94g formula (12 ), the molecular weight is 608, and the yield is 81.7%.
[0071] Product MS (m / e): 608, elemental analysis (C 46 h 28 N 2 ): theoretical value C: 90.76%, H: 4.64%, N:...
Embodiment 3
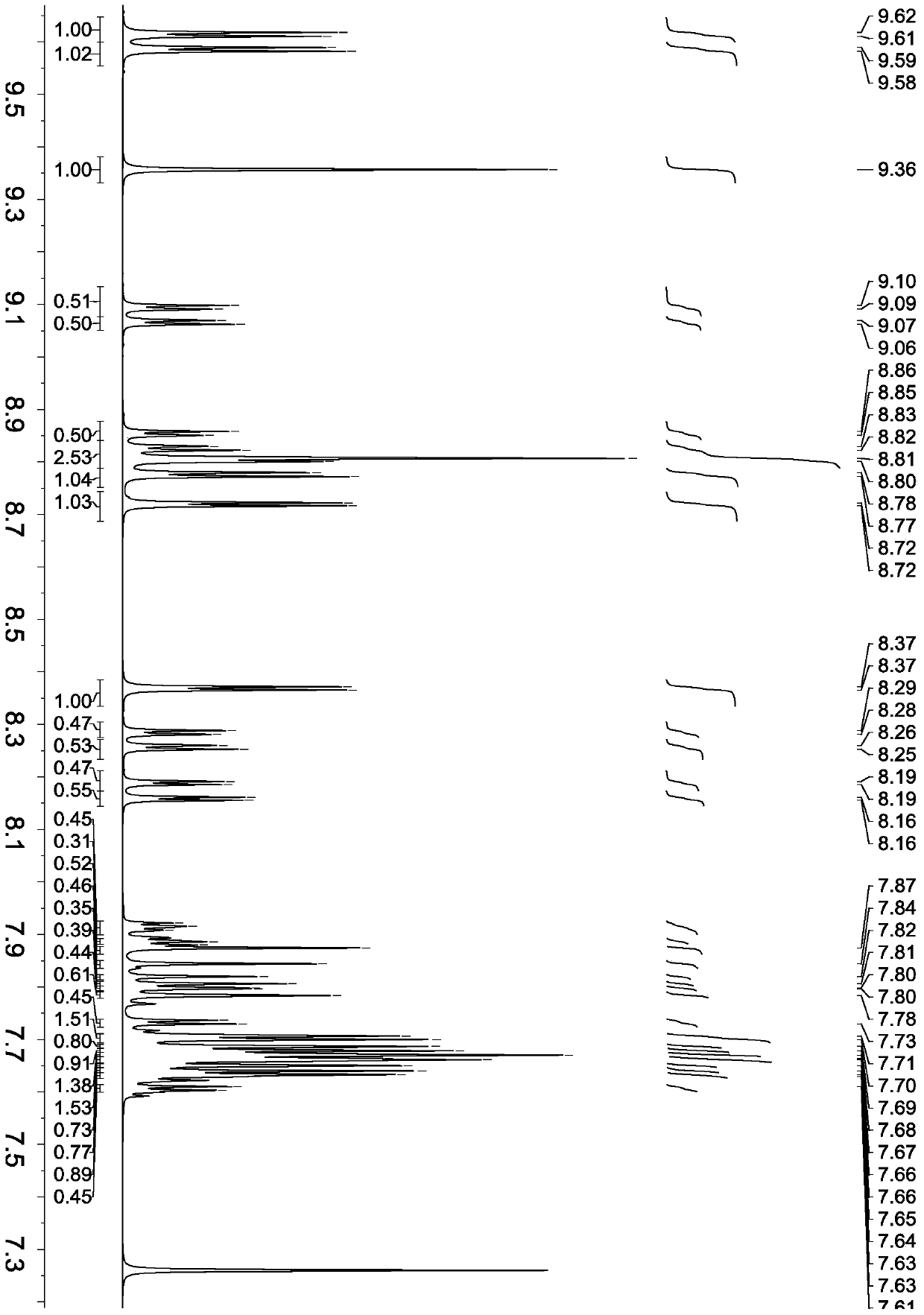
[0073] Synthesis of compound shown in formula (17)
[0074] The synthesis procedure is the same as in Example 2, except that 9,10-two (naphthalene-2-yl) anthracene-2-boronic acid is changed to 9-(naphthalene-2-yl)-10-(p-(naphthalene-2-yl ) phenyl) anthracene-2-boronic acid, and other reagents remain unchanged, the compound shown in formula (17) is obtained.
[0075] Product MS (m / e): 684, elemental analysis (C 52 h 32 N 2 ): theoretical value C: 91.20%, H: 4.71%, N: 4.09%; measured value C: 91.23%, H: 4.72%, N: 4.05%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| process yield | aaaaa | aaaaa |
| coating thickness | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com