Photoresist compositions and methods
A technology of photoresist and composition, applied in the field of chemical amplification material, negative tone development, photoresist composition and photolithography, can solve problems such as missing contact holes and pattern collapse
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
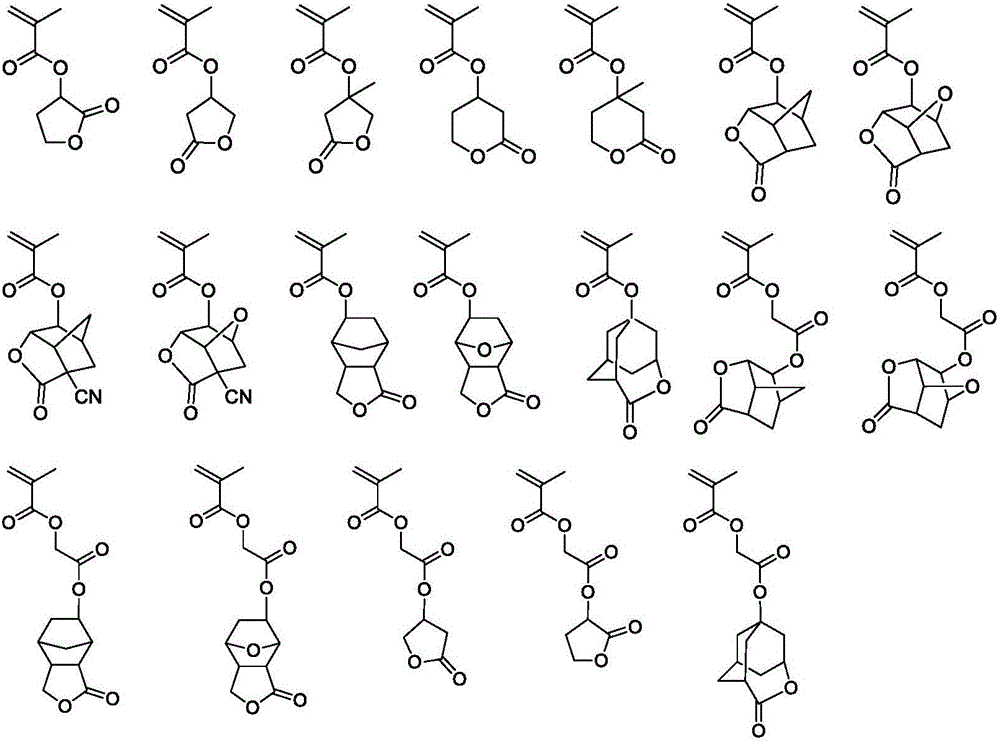
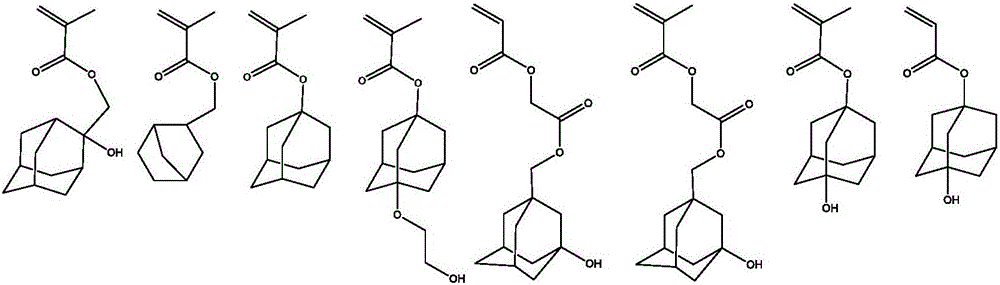
Image
Examples
example 1
[0100] Example 1: Synthesis of Monomer A
[0101]
[0102] Solution: Monomer A
[0103] A round bottom flask was charged with n-octylamine (10.0 g, 0.07743 mol) and ethylene carbonate (6.883 g, 0.0782 mol). The mixture was stirred at 100°C for 2 hours. The reaction mixture was cooled to room temperature and filtered. 16.3 g of product (starting material I) are obtained.
[0104] In a round bottom flask, starting material I (10.0 g, 0.0461 mol) and triethylamine (19.24 mL, 0.138 mol) were dissolved in 100 mL of anhydrous dichloromethane under nitrogen atmosphere. Methacryloyl chloride (5.82 mL, 0.0599 mol) was added dropwise at 0°C. The reaction mixture was allowed to warm slowly to room temperature and stirred at this temperature for 3 hours.
[0105] The reaction mixture was transferred to 100 mL deionized water, and the organic phase was washed with NH 4 Cl aqueous solution and deionized water were washed successively. The collected organic solutions were dried ove...
example 2
[0106] Example 2: Synthesis of Monomer B
[0107]
[0108] Solution: Monomer B
[0109]In a round bottom flask, 1-(tert-butoxycarbonyl)-4-piperidone (15.00 g, 0.0753 mmol) was dissolved in 300 mL of ether under nitrogen atmosphere. The resulting solution was cooled to -40°C and a 3M solution of ethylmagnesium bromide (32.64 mL, 0.0979 mmol) in diethyl ether was added. The reaction was stirred at -30-40°C for 30 minutes, then slowly warmed to room temperature and stirred for an additional 6 hours. by slowly adding H 2 O quenched the reaction and the resulting mixture was transferred to 200 mL deionized water, and the organic phase was washed with saturated NH 4 Cl was washed and the organic phase was washed with saturated NH 4 Cl and deionized water were washed successively. The collected organic solutions were dried over sodium sulfate, filtered and concentrated in vacuo. 12.2 g of product (starting material II) are obtained.
[0110] In a round bottom flask, startin...
example 3
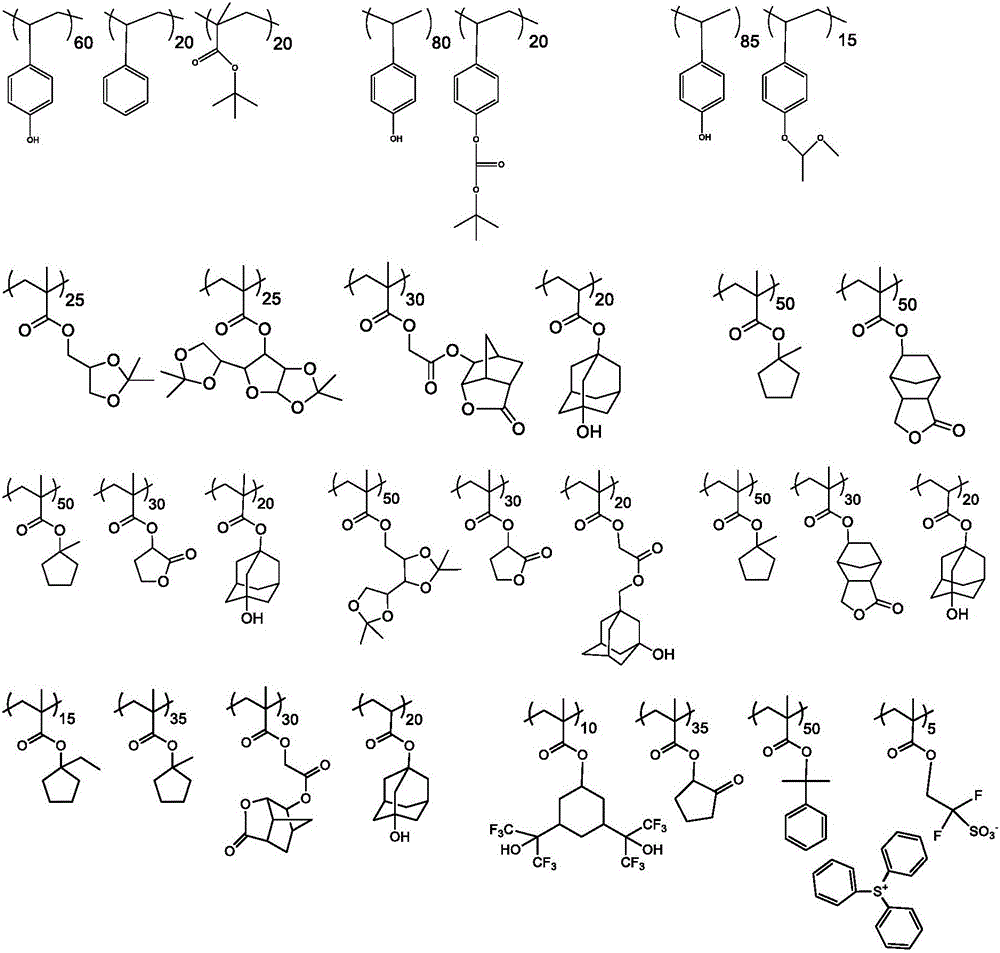
[0112] Example 3: Synthesis of Polymer B
[0113]
[0114] Scheme: Polymer B
[0115] In a round bottom flask, a series of monomers nBMA (2.85g), iBMA (15.17g) and TBPEMA (1.98g) were dissolved in 16.333g PGMEA with stirring at room temperature and degassed with nitrogen for 20 minutes. PGMEA (30.333 g) was fed into a Julabo reactor equipped with condenser and mechanical stirrer. After degassing with nitrogen for 20 minutes. The solvent in the Eulab reactor was heated to 80 °C. In another round bottom flask, initiator V601 (3.64 g) was dissolved in 5.47 g PGMEA and degassed with nitrogen for 20 minutes. The initiator solution was slowly added to the Ulab reactor and stirred for 15 minutes, and the monomer solution was fed dropwise into the Ulab reactor under a nitrogen atmosphere under strict stirring for 3 hours. After the monomer feed was complete, the reaction mixture was stirred at 80 °C for one hour. The reaction mixture was cooled to room temperature to achieve a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com