Method for dry preservation of biological tissue
A biological tissue and drying technology, applied in the field of implant materials, can solve the problems of easy calcification of tissues, cytotoxicity, long processing time, etc., and achieve the effect of eliminating side effects and high degree of proximity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
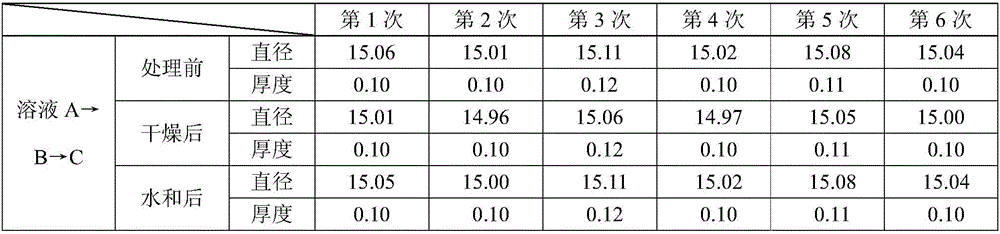
[0048] The glutaraldehyde-treated biological tissue (porcine pericardium was used in this experiment) was cut into three groups of similar size, 6 pieces in each group. Measure the size and record it. The pig pericardium was completely submerged in solution A for 12 hours, completely submerged in solution B for 12 hours after taking it out, and completely submerged in solution C for 12 hours after taking it out. Wherein solution A, B, the volume ratio of C and pig pericardium are all 80:1, and its distribution ratio of each group is as shown in Table 1.
[0049] Table 1
[0050] Solution combination Solution A Solution B Solution C glycerin 75% 80% 85% ethanol 25% 20% 15%
[0051] Test items:
[0052] 1. Measure and record the diameter and thickness of the pericardial slices before treatment, after drying and after hydration, and read after the data is stable for 5 seconds. The same pericardial slice was tested 6 times at different positi...
Embodiment 2-7
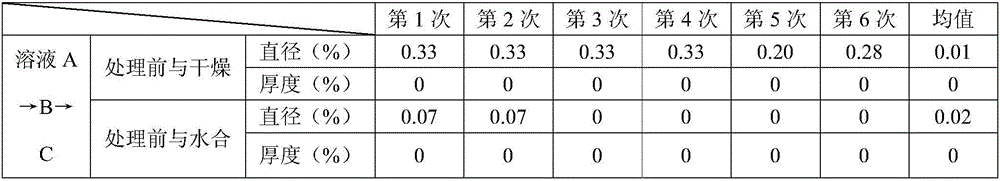
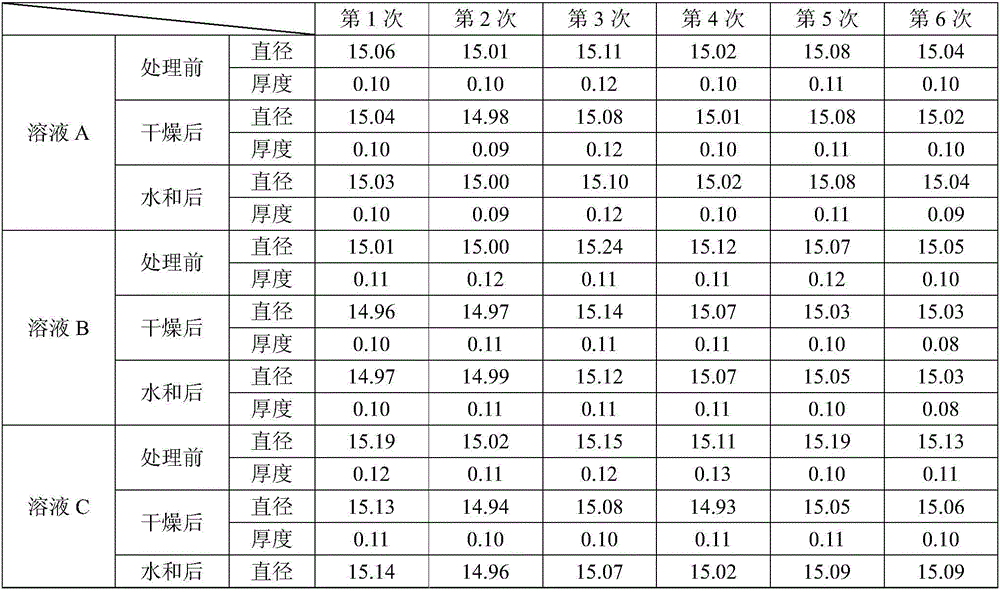
[0074] The components shown in Table 9 are used to prepare solutions A, B and C, and the order of solutions A→B→C is used to contact biological tissues. After testing, the diameter and thickness changes before treatment, after drying and after hydration The rates are shown in Table 10.
[0075] Table 9
[0076]
[0077] Table 10
[0078]
[0079] It can be seen from the above results that in Examples 1-7, the solutions A, B and C are used to contact the biological tissue in the order of solution A→B→C, which can make the size of the treated biological tissue closer to the size before dehydration. , so that the dry tissue is less susceptible to microorganisms; in the embodiments 1-7, compared with the other embodiments 2-7, the embodiment 1 can make the size of the biological tissue after treatment the closest to the size before dehydration.
[0080] The present invention has also verified other animal pericardium (such as cattle, sheep, kangaroos, etc.), aorta, mitral ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com