Quinoline acylhydrazone derivative-based fluorescence probe, and preparation method and application thereof
A quinoline acyl hydrazone, fluorescent probe technology, applied in fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., can solve problems such as metabolic disorders, disruption of zinc ion metabolic balance, many diseases, etc. Wide application value, strong selective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] A fluorescent probe based on quinoline acylhydrazone derivatives, characterized in that the quinoline acylhydrazone derivatives have the following structural formula:
[0027]
[0028] The preparation method of the fluorescent probe based on quinoline acylhydrazone derivatives comprises the following steps: dissolving 0.001-0.01mol of quinoline-8-carbaldehyde in 0.01-0.05L of absolute ethanol, and then adding 0.001-0.01 mol of acetylhydrazide, stirred at normal temperature and pressure for 4-6 hours, a large amount of solids were precipitated, filtered under reduced pressure, and the filter residue was washed with alcohol solution to obtain the fluorescent probe of the quinoline acylhydrazone derivative.
Embodiment 2
[0030] Synthesis of Quinolinylhydrazone Derivatives
[0031]
[0032] Dissolve 1.57 g of quinoline-8-carbaldehyde in 50 mL of absolute ethanol, then add 0.94 g of acetylhydrazide, stir and react at room temperature and pressure for 5 hours, a large amount of solid precipitates, filter under reduced pressure, wash the filter residue with absolute ethanol to obtain a white solid It is the target product, and the yield of the target product is 90.6%.
[0033] Quinoline acylhydrazone derivative nuclear magnetic resonance analysis results are as follows:
[0034] 1 H NMR (400 MHz, DMSO- d 6 ),δ(ppm): 10.19 (s, 1H, NH), 9.87 (s, 1H, NH),8.08-8.10 (d, 1H, Aryl-H), 7.93-7.95 (d, 1H, Aryl-H) , 7.74-7.76 (m, 1H, Aryl-H), 7.74-7.76 (m, 1H, Aryl-H), 7.58-7.65 (m, 2H, Aryl-H), 7.33-7.37 (m, 1H,Aryl- H), 7.02-7.09 (m, 2H, Aryl-H), 6.20-6.27 (m, 2H, Aryl-H), 6.03 (s, 1H, Aryl-H), 5.97 (s, 1H, Aryl-H) , 5.11 (s, 1H, NH), 4.60 (s, 1H, NH), 3.70 (s,3H, CH 3 ), 3.29 (s, 6H, 2CH 3 ), 2...
Embodiment 3
[0037] The structure of complexes formed by quinoline acylhydrazone derivatives with divalent zinc ions
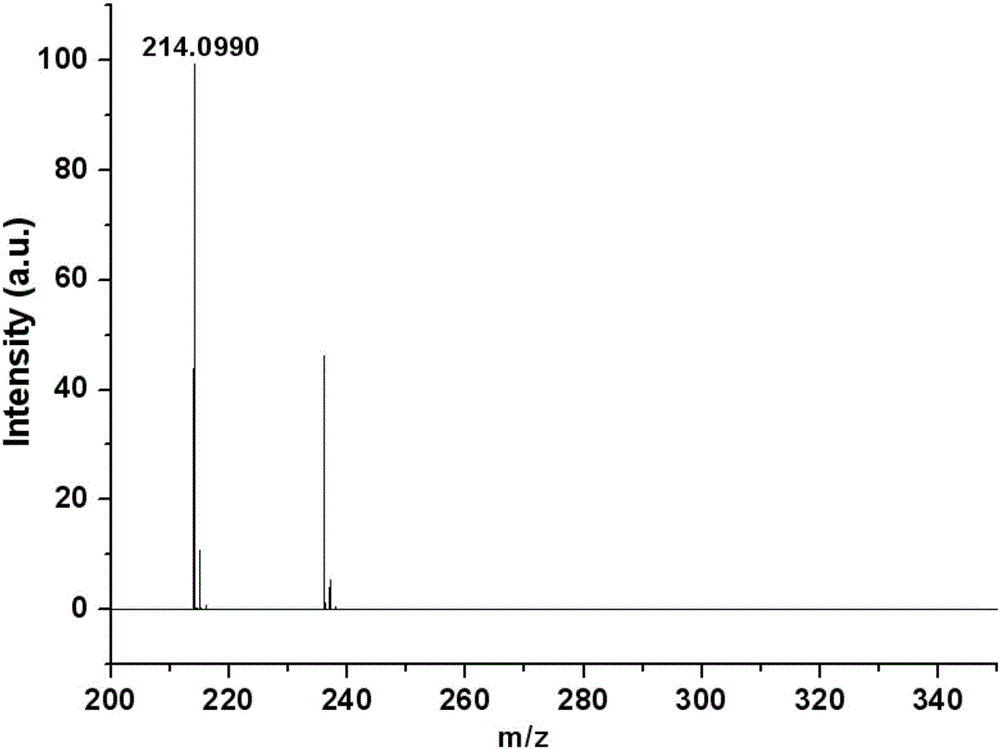
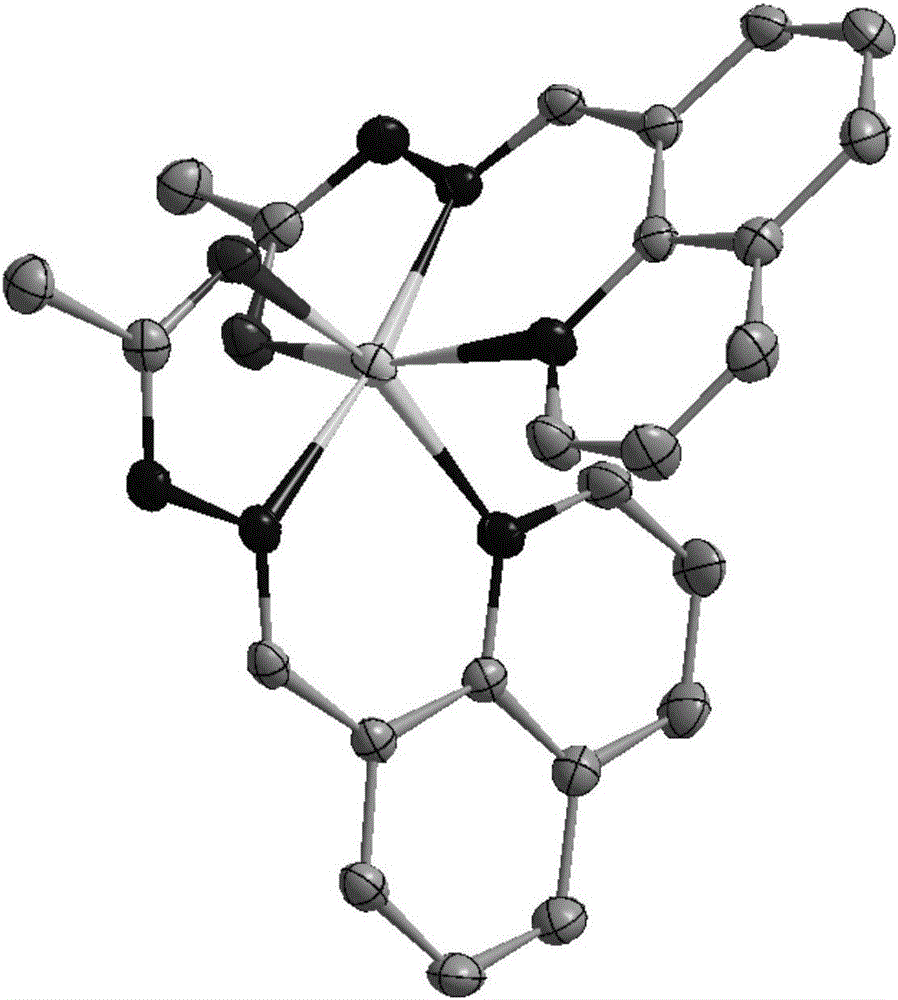
[0038] Dissolve 0.0213 g of quinoline acylhydrazone derivatives in 5 mL of absolute ethanol, then add 0.0110 g of zinc acetate dihydrate, stir and react at room temperature and pressure for 0.5 h, let stand at room temperature, and slowly evaporate the solvent to obtain the quinoline acylhydrazone zinc complex single crystal. For specific crystal structures, see image 3 . It shows that the quinoline acylhydrazone derivatives of the present invention can form stable complexes with zinc ions.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com