A bodipy-like dye that generates near-infrared fluorescence in situ and its preparation and application
A near-infrared and dye technology, applied in the direction of luminescent materials, organic dyes, azo dyes, etc., can solve the problems of poor labeling effect, large cell damage, and insufficient long emission wavelength
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
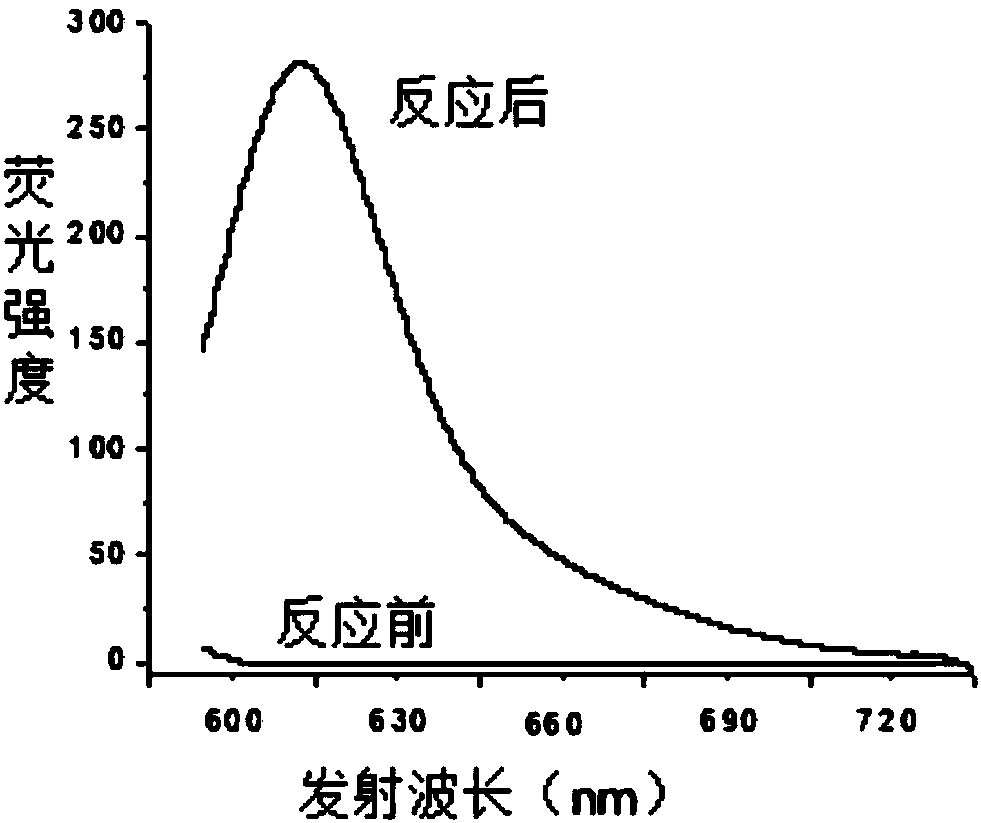
[0032] 1. Under nitrogen protection and stirring at room temperature, add 10 μL (0.0878 mmol) aniline dropwise to 2 mL of acetonitrile solution containing 25 mg (0.0438 mmol) of the compound of formula 1-1, stir at room temperature for 12 hours after the dropwise addition, stop the reaction, reduce Concentrate under reduced pressure to remove acetonitrile, and separate by column chromatography (the eluent is a mixture of petroleum ether and ethyl acetate with a volume ratio of 5:1) to obtain 11.2 mg of the compound of formula 2-1 as a red solid, with a yield of 57%. The reaction equation is as follows:
[0033]
[0034] The structural characterization data of the resulting product are: 1 H NMR (400MHz, CDCl 3 )δ:3.88(s,3H),6.35-6.36(d,J=3.88Hz,1H),6.43-6.45(d,J=4.8Hz,1H),6.94-6.95(d,J=4.92Hz,1H ),6.98-7.01(m,2H),7.24-7.26(m,4H),7.30-7.44(m,3H),8.12(s,1H); 13 C NMR (100MHz, CDCl 3 )δ: 55.43, 111.66, 113.87, 116.78, 117.29, 122.07, 122.50, 126.00, 126.17, 129.82, 131.73, ...
Embodiment 2
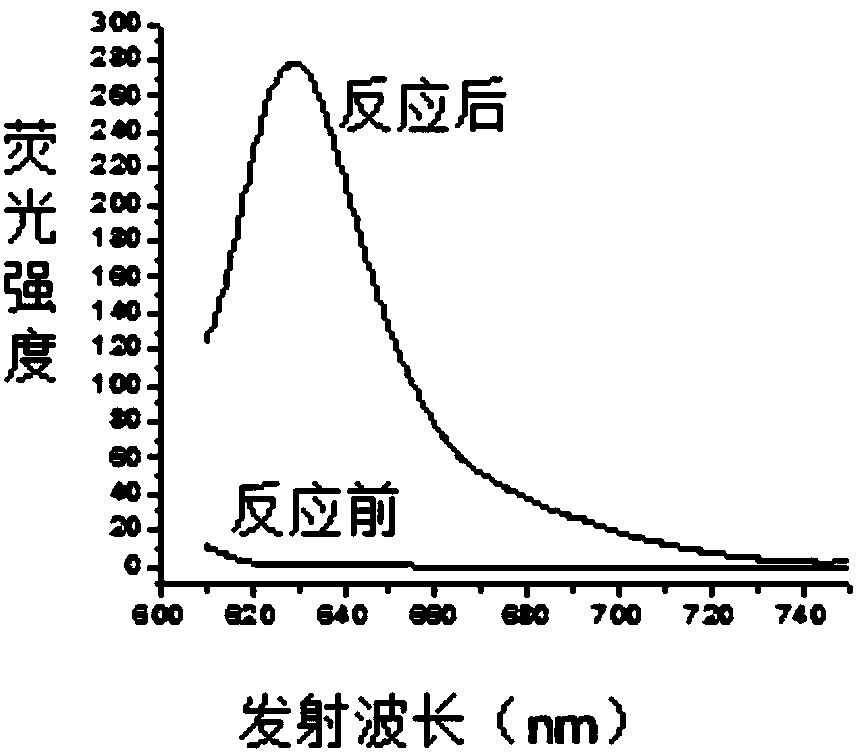
[0039] 1. Under nitrogen protection and stirring at room temperature, add 5.78 μL (0.052 mmol) of phenylacetylene dropwise to 2 mL of acetonitrile solution containing 20 mg (0.0439 mmol) of the compound of formula 1-1, stir at room temperature for 2 hours after the dropwise addition, and stop the reaction , concentrated under reduced pressure to remove acetonitrile, separated by column chromatography (eluent is toluene), obtained 11 mg of red solid formula 2-2 compound, and its yield was 68%. The reaction equation is as follows:
[0040]
[0041] The structural characterization data of the resulting product are: 1 H NMR (400MHz, CDCl 3 )δ: 3.9 (s, 3H) 6.53-6.54 (d, J = 4.28Hz, 1H), 6.72-6.73 (d, J = 4.32Hz, 1H), 6.82-6.83 (d, J = 4.24Hz, 1H) ,6.91-6.90(d,J=4.24Hz,1H),7.03-7.05(m,2H),7.37-7.39(m,2H),7.49-7.47(m,2H),7.69-7.66(m,2H) ; 13 C NMR (100MHz, CDCl 3 )δ: 161.96, 142.79, 137.77, 136.12, 135.79, 132.35, 131.47, 130.97, 130.70, 129.60, 129.04, 128.40, 125.63, 123.97,...
Embodiment 3
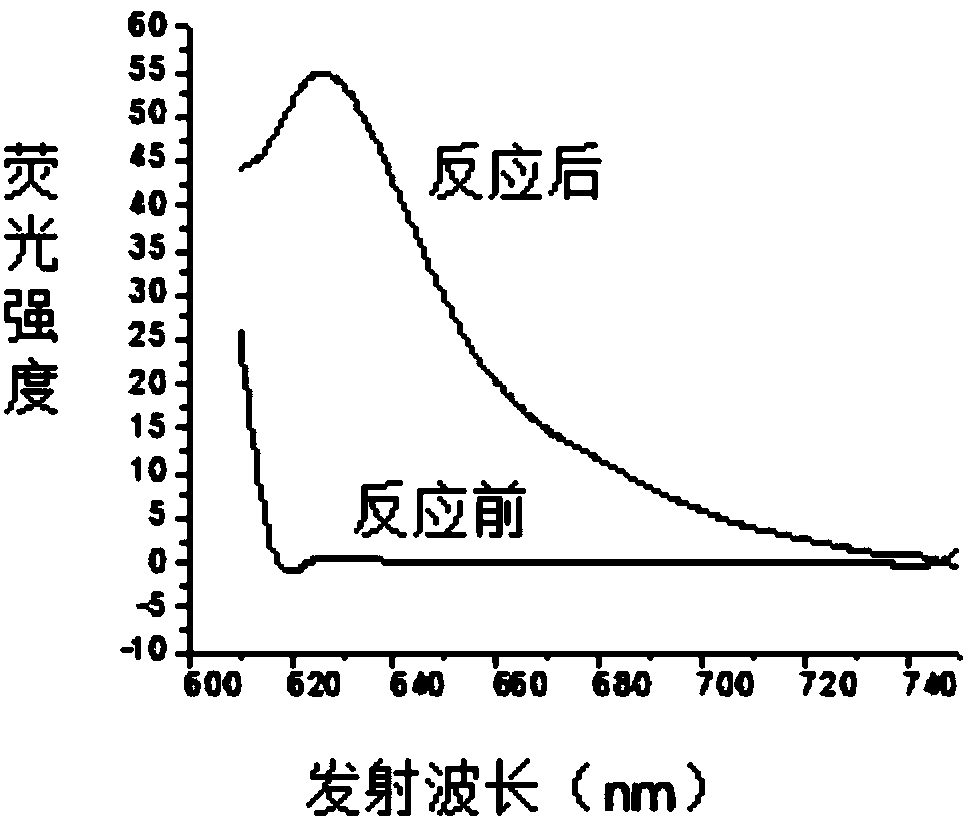
[0046] 1. Under nitrogen protection and stirring at room temperature, add 7 mg (0.045 mmol) of the compound of formula a-1 dropwise to 2 mL of acetonitrile solution containing 21 mg (0.045 mmol) of the compound of formula 1-1, and stir at room temperature for 12 hours after the addition is complete. Stop the reaction, concentrate under reduced pressure to remove acetonitrile, separate by column chromatography (eluent is the mixed solution of sherwood oil and ethyl acetate with a volume ratio of 5:1), obtain 11 mg of red solid formula 2-3 compound, and its yield is 52%, the reaction equation is as follows:
[0047]
[0048] The structural characterization data of the resulting product are: 1 H NMR (400MHz, CDCl 3 )δ:3.88(s,3H),3.92(s,3H),6.37-6.38(d,J=3.92Hz,1H),6.49-6.50(d,J=3.92Hz,1H),6.55-6.54(d ,J=4.88Hz,1H),6.98-7.01(m,3H),7.29-7.27(m,2H),7.42-7.40(d,J=8.64Hz,2H),8.07-8.09(d,J=8.6 Hz,2H),8.21(s,1H); 13 C NMR (100MHz, CDCl 3 )δ: 166.12, 161.02, 156.96, 141.72, 135.4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com