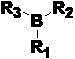Efficient anti-poisoning Karstedt catalyst and synthesis and application in hydrosilylation reaction
A hydrosilylation reaction and catalyst technology, applied in catalytic reactions, physical/chemical process catalysts, organic compounds/hydrides/coordination complex catalysts, etc., can solve the problem of low yield, increased platinum consumption, and increased production Cost and other issues, to achieve the effect of improving activity and stability, reducing the amount of Pt usage, and saving costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1. Synthesis of highly efficient antipoisoning Karstedt catalysts.
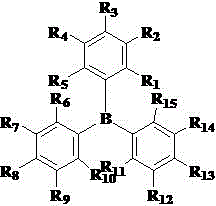
[0033] Under the protection of nitrogen, add 4 mmol of Karstedt catalyst (divinyltetramethyldisiloxane with Pt) into a 250ml three-necked flask and add 100ml of toluene solution dissolved in 16mmol of triphenylborane, and reflux at 80°C for 2h . After cooling to room temperature, a pale yellow catalyst solution was obtained.
Embodiment 2
[0034] Example 2. Synthesis of highly efficient antipoisoning Karstedt catalysts.
[0035] Under the protection of nitrogen, add 4 mmol of Karstedt catalyst (divinyltetramethyldisiloxane with Pt) into a 250ml three-necked flask and add 100 ml of toluene solution dissolved in 4mmol of triphenylborane, and reflux at 80°C for 2h . After cooling to room temperature, a pale yellow catalyst solution was obtained.
Embodiment 3
[0036] Example 3. Synthesis of highly efficient antipoisoning Karstedt catalysts.
[0037] Under nitrogen protection, add 4 mmol of Karstedt catalyst (divinyltetramethyldisiloxane with Pt) into a 250ml three-necked flask and add 100 ml of dichloromethane dissolved in 4mmol of tris(pentafluorophenyl)borane Solution, reflux at 60 degrees for 12h. After cooling to room temperature, a pale yellow catalyst solution was obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com