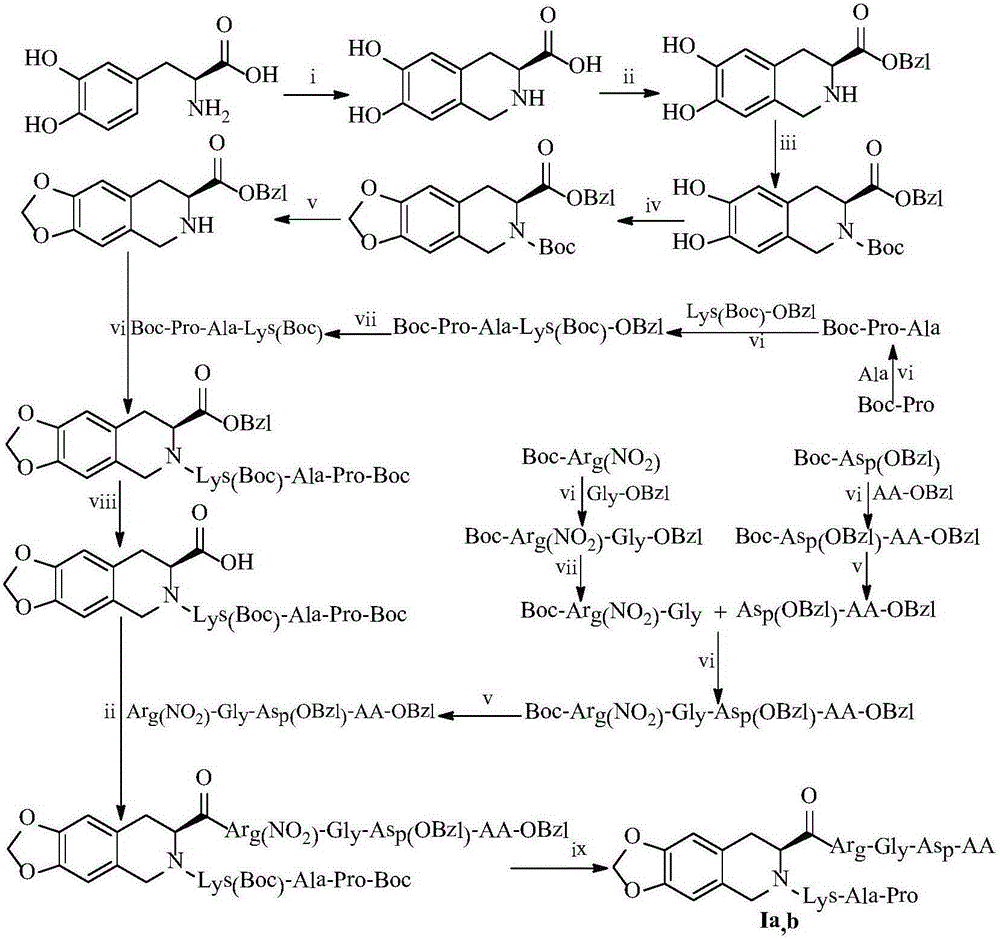
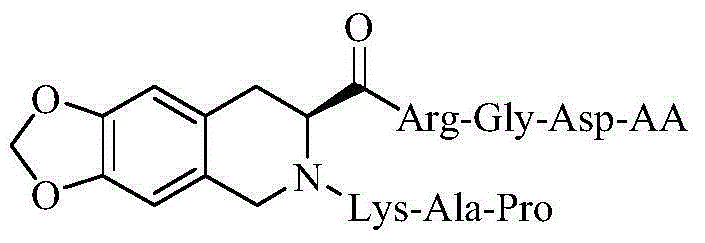
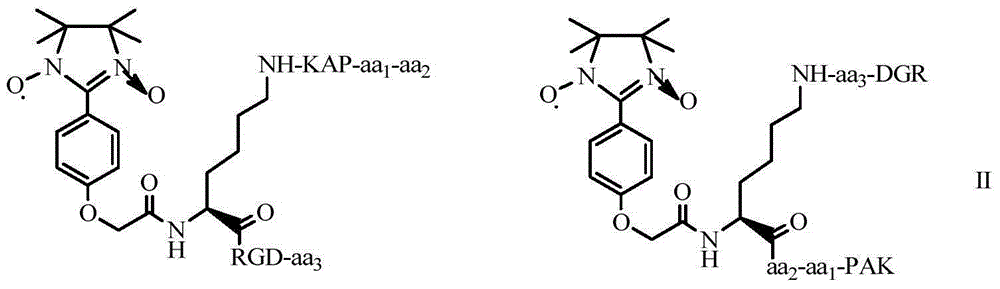
N-(PAK)-2,3-dioxide-isoquinoline-7-formyl-RGDV/F, synthesis, activity and application thereof
A technology of dioxyisoquinoline and dihydroxyisoquinoline, which is applied in the field of biomedicine and can solve problems such as no effective drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1 Preparation of (7S)-5,6,7,8-tetrahydro-2,3-dihydroxyisoquinoline-7-carboxylic acid
[0029] To 988 mg (0.005 mmol) of (S)-3,4-dihydroxy-Phe was sequentially added 5.9 mL of H 2 O, 0.7mL 32% hydrochloric acid, stir evenly, after complete dissolution, add 1.2mL 40% formaldehyde solution, react at room temperature for 6 hours. TLC (dichloromethane:methanol, 10:1) showed that (S)-3,4-dihydroxy-Phe disappeared, and saturated sodium bicarbonate was added dropwise in ice bath to make the pH to 5, and a large amount of colorless precipitates were precipitated. Filtration yielded 1.01 g (95%). Mp 281-286℃, ESI-MS(m / z): 210[M+H] + .
Embodiment 2
[0030] Example 2 Preparation of (7S)-5,6,7,8-tetrahydro-2,3-dihydroxyisoquinoline-7-carboxylic acid benzyl ester
[0031] 1 g (4.08 mmol) of (7S)-5,6,7,8-tetrahydro-2,3-dihydroxyisoquinoline-7-carboxylic acid, 1.02 g of p-toluenesulfonic acid, 8 mL of benzyl alcohol and 8 mL of n-hexane The mixture was heated and stirred at 90-95°C for 36h to make it clear. After the reaction mixture was cooled to room temperature, a large amount of diethyl ether was added to precipitate a large amount of colorless solid, which was filtered, washed with diethyl ether, and dried to obtain 1.08 g (77%) of the title compound as a colorless powder. ESI-MS(m / z):300[M+H] + .
Embodiment 3
[0032] Example 3 Preparation of (7S)-N-tert-butoxycarbonyl-5,6,7,8-tetrahydro-2,3-dihydroxyisoquinoline-7-carboxylic acid benzyl ester
[0033] 1g (3.32mmol) (7S)-benzyl 5,6,7,8-tetrahydro-2,3-dihydroxyisoquinoline-7-carboxylate was dissolved in 50ml THF under ice bath, and then 0.93g Boc 2 O, adjust the pH to 8 with NMM, react at room temperature for 48h, and monitor the completion of the reaction by TLC. The reaction compound was concentrated to dryness under reduced pressure, and the yellow oil was purified by column chromatography (petroleum ether / acetone: 6 / 1-1 / 1) to obtain 243 mg (35%) of the title compound as a yellow powder. ESI-MS(m / z): 400[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com