Polypeptide-modifying polyamide-amine branch type polymer and preparation method and application thereof
A technology of polypeptide modification and polyamide, which is applied in the field of biomedical antibacterial materials, can solve the problems of differences in antibacterial properties and cytotoxicity, complicated preparation and application, etc., and achieves the effects of firm adsorption, simple preparation process, and easy industrial production.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
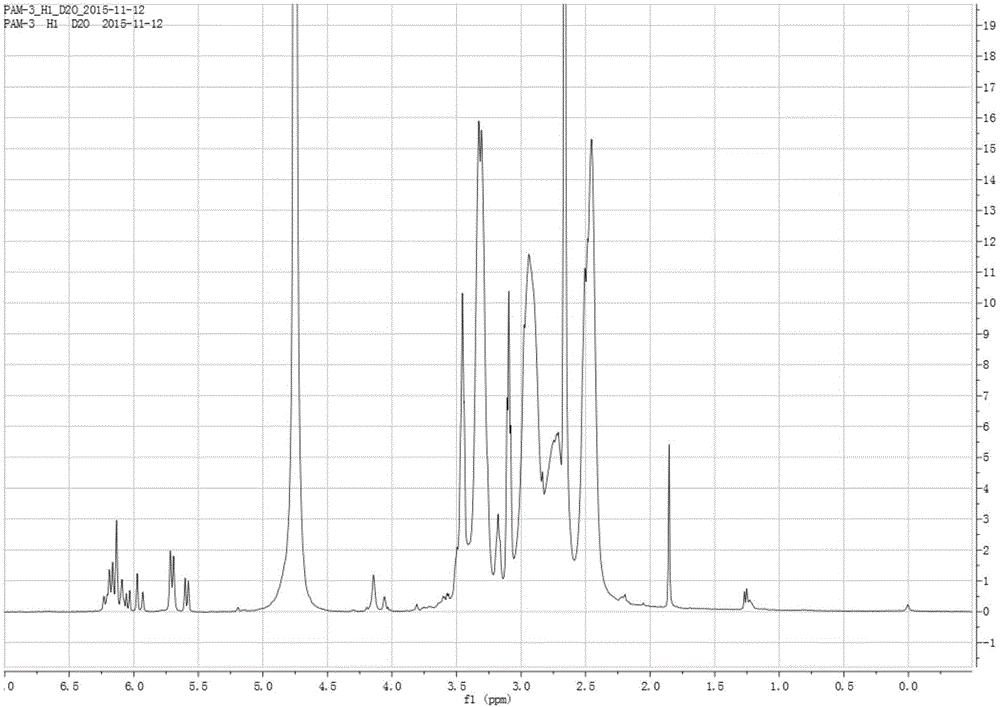
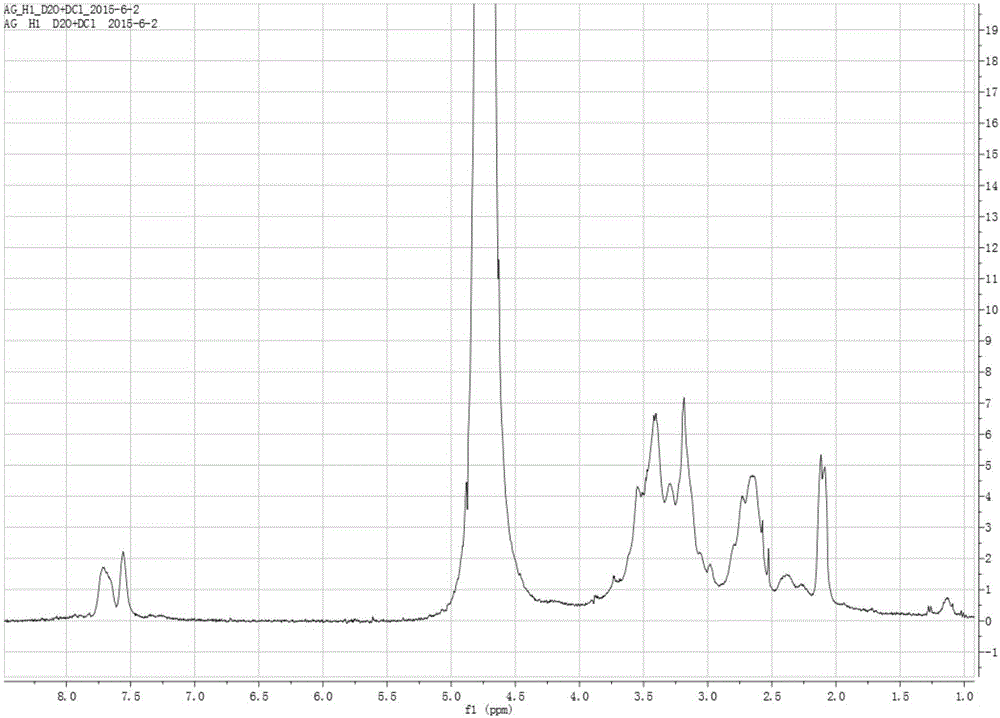
Image
Examples
Embodiment 1
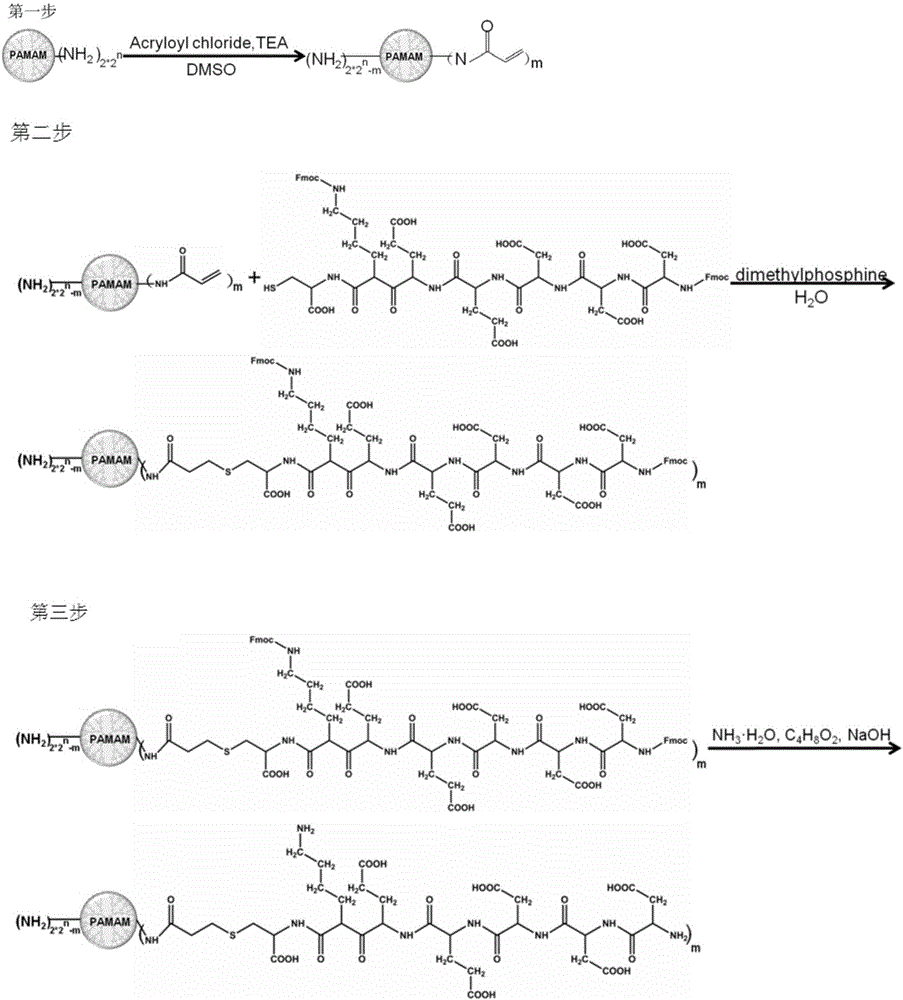
[0049] In this example, the synthetic route of the 4th generation polyamide-amine type dendritic polymer whose terminal group is modified by polypeptide is as follows: figure 1 As shown, the specific preparation method is as follows:
[0050] (1) Synthesis of polyamide-amine dendrimers whose end groups are acryloyl groups
[0051] ① Prepare the first solution
[0052] At room temperature and normal pressure, 1.0 g of the polyamide-amine dendrimers (G4PAMAM-NH 2 ) was dissolved in 1 mL of anhydrous dimethyl sulfoxide to form the first solution, in which G4PAMAM-NH 2 The concentration is about 1g / mL;
[0053] ② Prepare the second solution
[0054] Dissolve triethylamine (TEA) in the above-mentioned first solution at room temperature and normal pressure to form a second solution; the TEA and the G4PAMAM-NH in the first solution 2 The molar ratio of molecules is 3:1;
[0055] ③Synthesis
[0056] Under the protection of argon, add acryloyl chloride (Acryloyl chloride) to the...
Embodiment 2
[0069] In this example, the synthetic route of the 5th generation polyamide-amine dendrimers whose terminal groups are modified by polypeptides is as follows: figure 1 As shown, the specific preparation method is as follows:
[0070] (1) Synthesis of polyamide-amine dendrimers whose end groups are acryloyl groups
[0071] ① Prepare the first solution
[0072] At room temperature and normal pressure, 2.5g of the polyamide-amine dendrimers (G5PAMAM-NH 2 ) was dissolved in 2mL of anhydrous dimethyl sulfoxide to form the first solution, in which G5PAMAM-NH 2 The concentration is about 1.25g / mL;
[0073] ② Prepare the second solution
[0074] Dissolve triethylamine (TEA) in the above-mentioned first solution at room temperature and normal pressure to form a second solution; the TEA and the G5PAMAM-NH in the first solution 2 The molar ratio of molecules is 3.3:1;
[0075] ③Synthesis
[0076] Under the protection of argon, add acryloyl chloride (Acryloyl chloride) to the secon...
Embodiment 3
[0089] In this example, the synthetic route of the 6th generation polyamide-amine dendrimers whose end groups are modified by polypeptides is as follows: figure 1 As shown, the specific preparation method is as follows:
[0090] (1) Synthesis of polyamide-amine dendrimers whose end groups are acryloyl groups
[0091] ① Prepare the first solution
[0092] At room temperature and normal pressure, the polyamide-amine dendrimers (G6PAMAM-NH 2 ) was dissolved in 3mL of anhydrous dimethyl sulfoxide to form the first solution, in which G6PAMAM-NH 2 The concentration is about 1.33g / mL;
[0093] ② Prepare the second solution
[0094] Dissolve triethylamine (TEA) in the above-mentioned first solution at room temperature and normal pressure to form a second solution; the TEA and the G6PAMAM-NH in the first solution 2 The molar ratio of molecules is 3.4:1;
[0095] ③Synthesis
[0096]Under the protection of argon, add acryloyl chloride (Acryloyl chloride) to the second solution at ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| water content | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com