Separation and purification method for 2,6-diisopropylnaphthalene
A technology of diisopropylnaphthalene and a purification method, which is applied in the field of separation and purification of 2,6-diisopropylnaphthalene, can solve problems such as complex process, low product purity, and difficulty in industrial application, and achieve simple process and guaranteed product Purity, the effect of reducing the amount of solvent
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
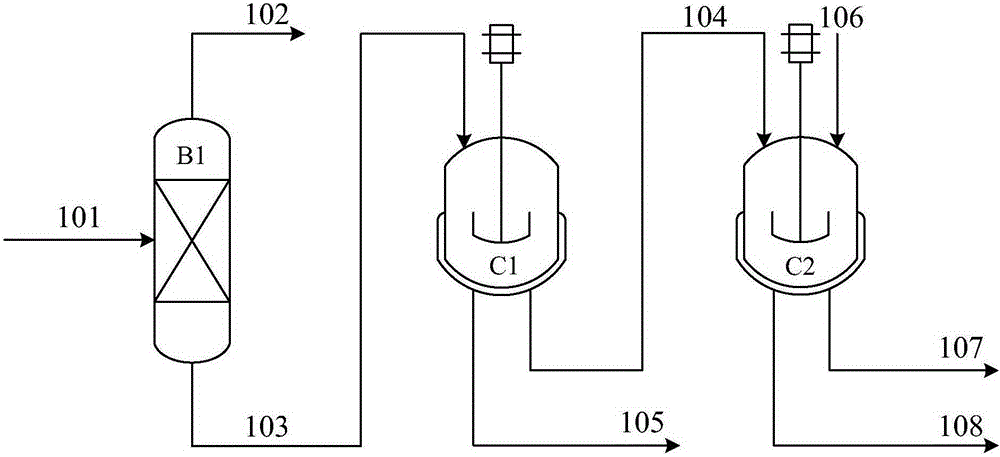
[0026] use figure 1 In the flow process shown, the stream (101) is a mixed material liquid containing 2,6-diisopropylnaphthalene, and its composition in weight percentage is: 19.28% of naphthalene, 49.03% of monoisopropylnaphthalene, 2,6- Diisopropylnaphthalene 10.77%, 2,7-diisopropylnaphthalene 11.12%, other heavy components such as diisopropylnaphthalene and triisopropylnaphthalene 9.8%.
[0027] The number of theoretical plates of rectification column B1 is 30, the stream (101) enters from the 20th theoretical plate, the operating pressure is 10KPa, the reflux ratio is 0.5, the temperature at the top of the tower is 157.07°C, and the temperature at the bottom of the tower is 222.27°C. After separation, the stream (103) does not contain naphthalene, and the composition of 2,6-diisopropylnaphthalene is 34.74% by weight; the stream (103) enters the crystallizer C1, the crystallization temperature is -15°C, and the crystallization time is 6hr. After crystallization and filtra...
Embodiment 2
[0029] Adopt the same flow process and raw material composition as embodiment 1. The number of theoretical plates of rectification column B1 is 20, the stream (101) enters from the 12th theoretical plate, the operating pressure is 30KPa, the reflux ratio is 1.2, the temperature at the top of the tower is 193.89°C, and the temperature at the bottom of the tower is 262.08°C. The stream (103) does not contain naphthalene, and the composition of 2,6-diisopropylnaphthalene in weight percentage is 34.74%; the stream (103) enters the crystallizer C1, the crystallization temperature is -20°C, and the crystallization time is 7hr. After suspended crystallization and filtration, the purity of 2,6-diisopropylnaphthalene in weight percent in the crystal of stream (104) is 73.09%; Stream (104) continues to enter crystallizer C2, and solvent is ethanol, and crystallization temperature is 0 ℃, the crystallization time is 7hr, after solvent crystallization, methanol washing, and filtration, dr...
Embodiment 3
[0031] use figure 1 In the flow process shown, the stream (101) is a mixed material liquid containing 2,6-diisopropylnaphthalene, and the composition in weight percentage is: naphthalene 11.39%, monoisopropylnaphthalene 31.53%, 2,6- Diisopropylnaphthalene 20.18%, 2,7-diisopropylnaphthalene 21.19%, other heavy components such as diisopropylnaphthalene and triisopropylnaphthalene 15.71%.
[0032] The number of theoretical plates in rectification column B1 is 25, the stream (101) enters from the 17th theoretical plate, the operating pressure is 5KPa, the reflux ratio is 0.9, the temperature at the top of the tower is 138.27°C, and the temperature at the bottom of the tower is 199.99°C. The stream (103) does not contain naphthalene, and the composition of 2,6-diisopropylnaphthalene in weight percentage is 36.04%; the stream (103) enters the crystallizer C1, the crystallization temperature is -10°C, and the crystallization time is 5hr. After suspended crystallization and filterin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com