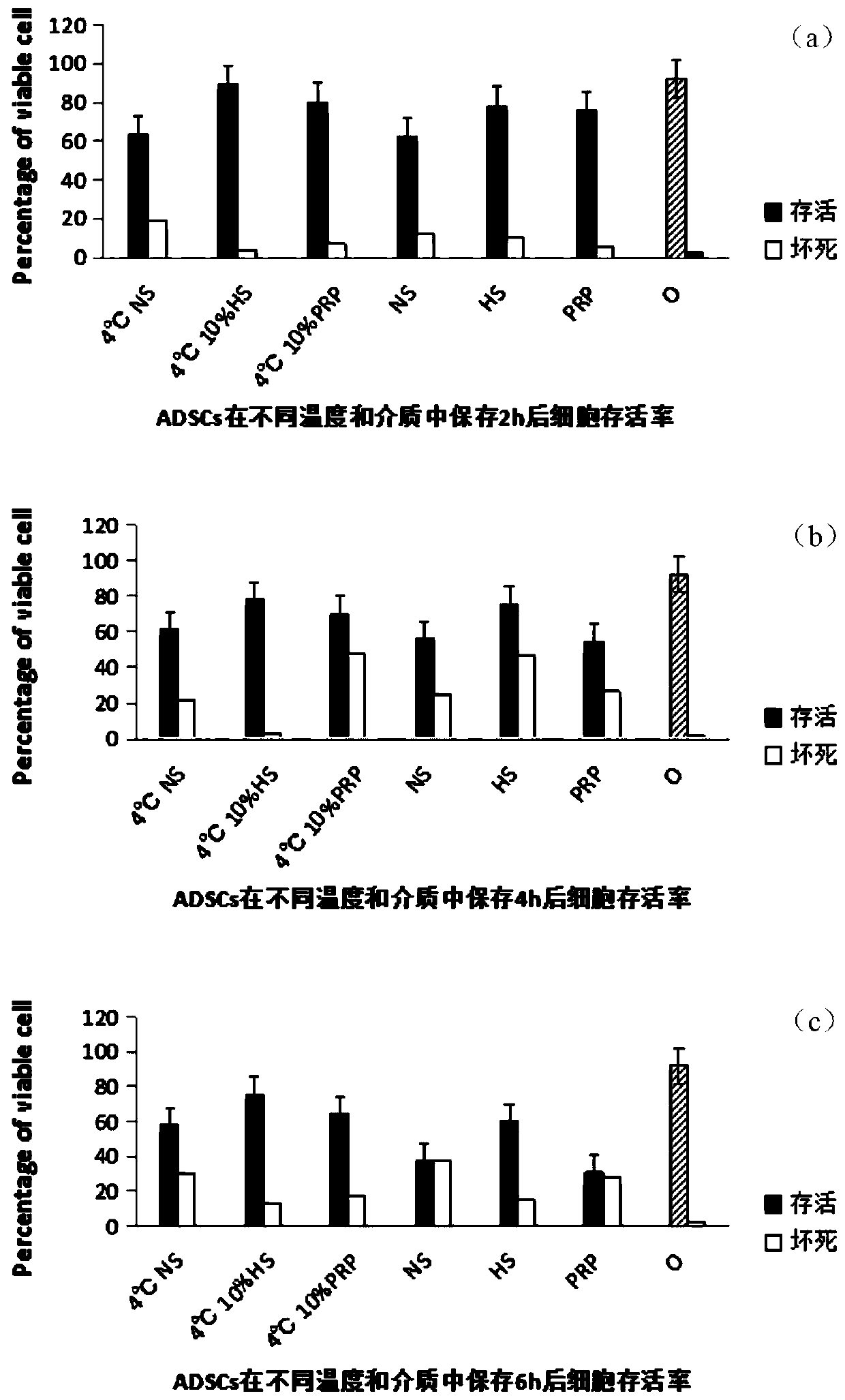
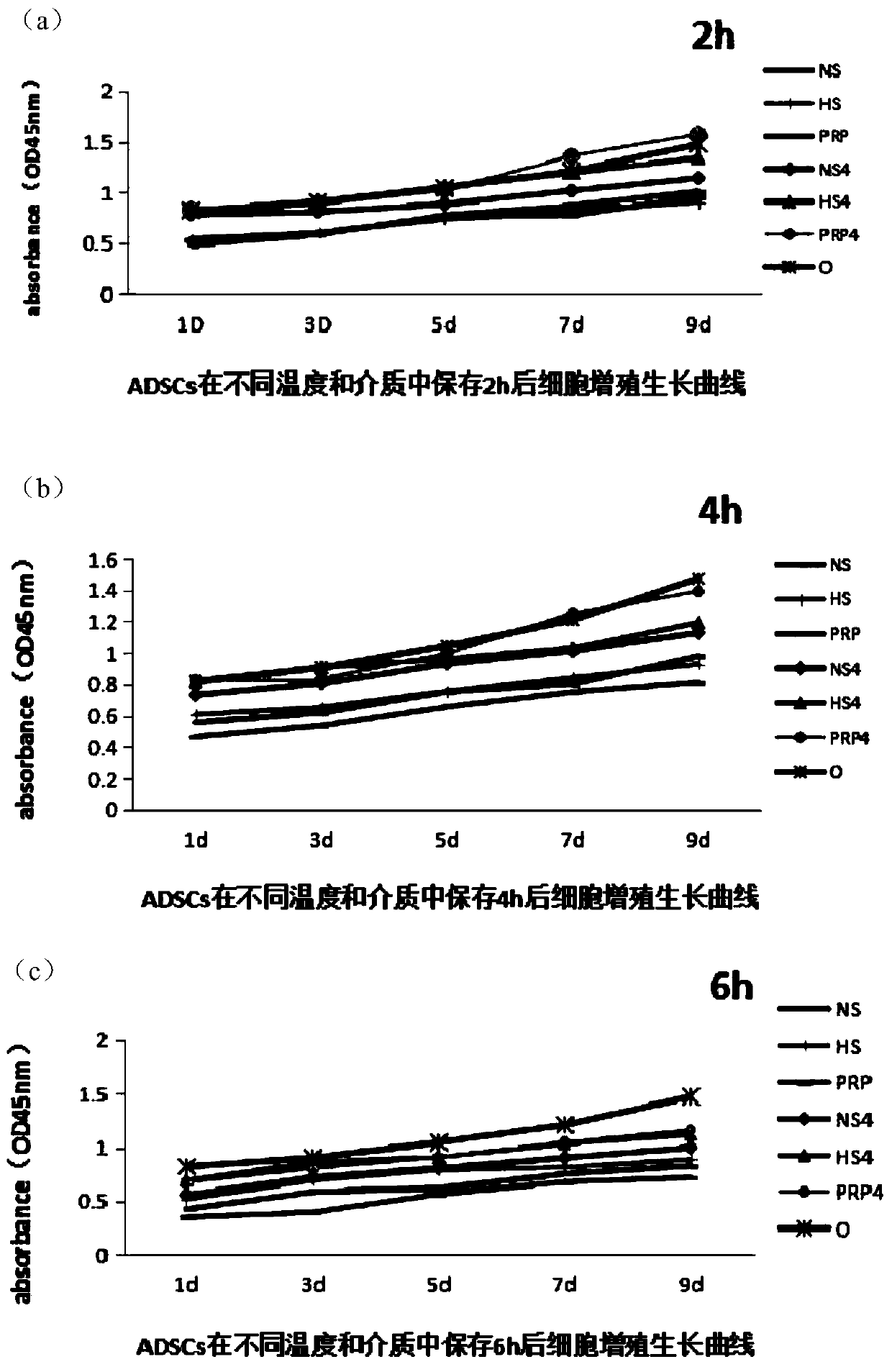
Clinical ready-to-use active preservation method for adipose-derived stem cells
A preservation method and stem cell technology, applied in the field of cell preservation media
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] A method for clinical ready-to-use active preservation of adipose-derived stem cells, comprising the following steps:
[0037] (1) Separation of adipose-derived stem cells and grouping of temperature and cell preservation solution:
[0038] ①Adipose tissue from 6 cases of 25-40-year-old female thigh and abdomen liposuction was taken, and adipose-derived stem cells were extracted by the same 0.1% collagenase digestion and centrifugation method, planted and subcultured at the same cell density, and P2 generation cells were obtained ;
[0039] ②Take well-growing P2 generation cells, wash each culture bottle with PBS, use 1mL 0.25% trypsin, place in a 37°C incubator to digest for 5min, add 3mL PBS buffer to dilute, centrifuge at 300g for 10min, discard the supernatant, and add 1mL PBS buffer, after blowing gently, the cell counting plate is counted and recorded;
[0040] ③Prepare 14 test tubes in the ultra-clean bench, each with 5×10 5 The cell density was suspended in 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com