Phytosterol compound lipid-decreasing formula and preparation method for tablet thereof
The technology of phytosterol and tablet is applied in the field of phytosterol compound lipid-lowering formula and tablet preparation field, which can solve the problems of single action mode, poor tablet stability, poor solubility of phytosterol and the like, and achieves improved effect, The effect of improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
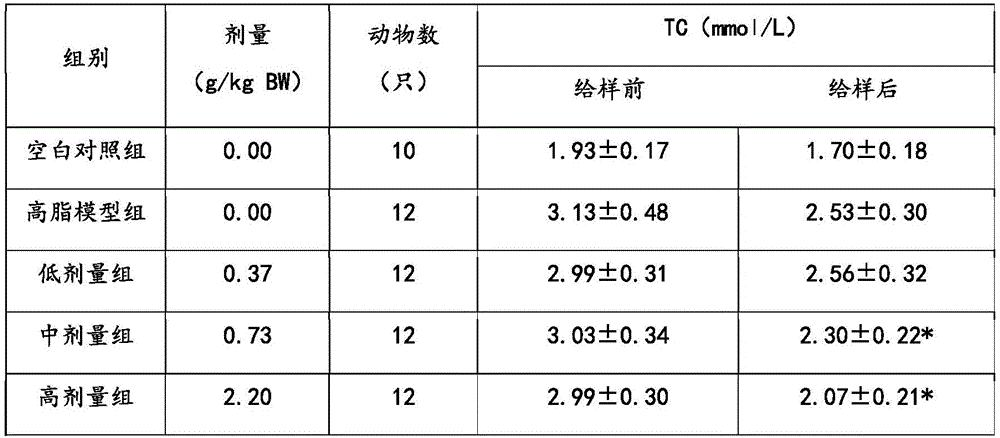
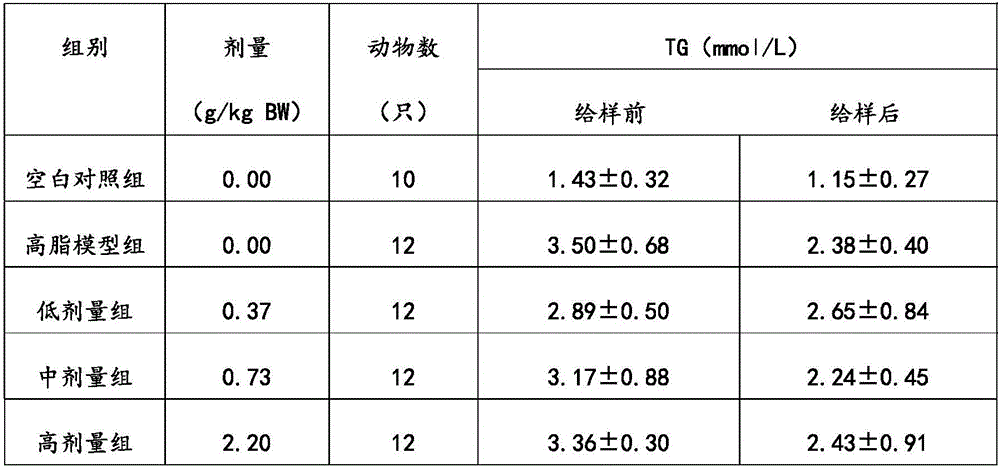
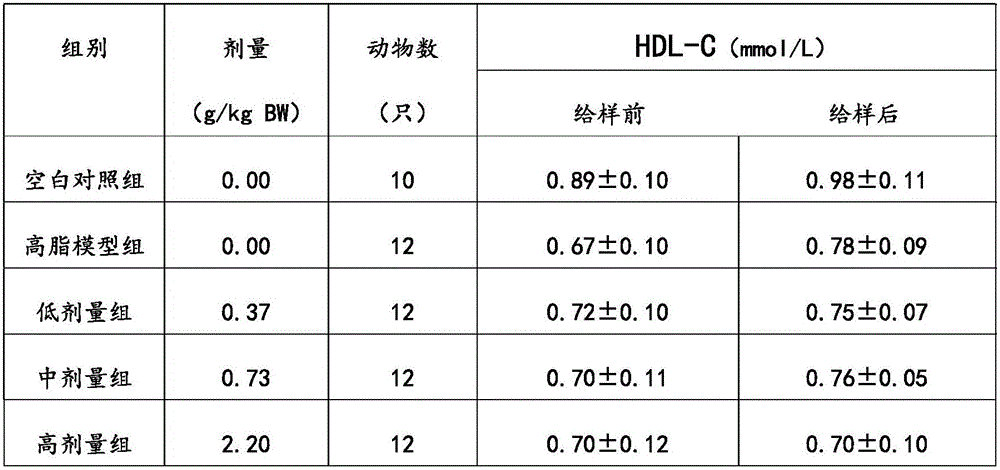
[0028] The phytosterol compound lipid-lowering formula of the present embodiment includes the following components: by weight, 15 parts of phytosterol, 5 parts of soybean peptide, 5 parts of β-glucan, 1 part of lecithin, and 10 parts of mannitol , 1 part of maltodextrin, 0.01 part of sucralose and 5 parts of microcrystalline cellulose.
[0029] The tablet preparation method of the phytosterol compound lipid-lowering prescription of the present embodiment comprises the following steps:
[0030] Step 1, take 15 parts of phytosterol, 5 parts of soybean peptide, 5 parts of β-glucan, 1 part of lecithin, 10 parts of mannitol, 1 part of maltodextrin, 0.01 part of sucralose and 5 parts of microcrystalline cellulose Mix and stir evenly to obtain a mixture;
[0031] Step 2, adding 50% ethanol solution to the mixture and performing wet granulation with a wet granulator to obtain wet granules;
[0032] Step 3, drying the wet granules in a hot air circulation oven at 50°C to obtain dry g...
Embodiment 2
[0036] The phytosterol compound lipid-lowering formula of the present embodiment includes the following components: in parts by weight, 30 parts of phytosterols, 20 parts of soybean peptides, 15 parts of β-glucan, 10 parts of lecithin, and 30 parts of mannitol , 10 parts of maltodextrin, 0.5 parts of sucralose and 15 parts of microcrystalline cellulose.
[0037] The tablet preparation method of the phytosterol compound lipid-lowering prescription of the present embodiment comprises the following steps:
[0038] Step 1: Take 30 parts of phytosterol, 20 parts of soybean peptide, 15 parts of β-glucan, 10 parts of lecithin, 30 parts of mannitol, 10 parts of maltodextrin, 0.5 parts of sucralose and microcrystalline cellulose 15 parts of the element were mixed and stirred evenly to obtain a mixture;
[0039] Step 2, adding 60% ethanol solution to the mixture and using a wet granulator to carry out wet granulation to obtain wet granules;
[0040] Step 3, drying the wet granules in ...
Embodiment 3
[0044] The phytosterol compound lipid-lowering prescription of the present embodiment includes the following components: by weight, 70 parts of phytosterols, 50 parts of soybean peptides, 30 parts of β-glucan, 20 parts of lecithin, and 70 parts of mannitol , 30 parts of maltodextrin, 1 part of sucralose and 30 parts of microcrystalline cellulose.
[0045] The tablet preparation method of the phytosterol compound lipid-lowering prescription of the present embodiment comprises the following steps:
[0046] Step 1: Take 70 parts of phytosterol, 50 parts of soybean peptide, 30 parts of β-glucan, 20 parts of lecithin, 70 parts of mannitol, 30 parts of maltodextrin, 1 part of sucralose and microcrystalline cellulose Mix and stir 30 parts of plain ingredients evenly to obtain a mixture;
[0047] Step 2, adding 75% ethanol solution to the mixture and using a wet granulator to carry out wet granulation to obtain wet granules;
[0048] Step 3, drying the wet granules in a hot air circ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com