Animophenoxy phthalonitrile resin monomer and synthetic method thereof
A technology of aminophenoxy phthalonitrile resin and synthesis method, applied in chemical instruments and methods, preparation of carboxylic acid nitrile, preparation of organic compounds, etc., can solve problems such as less obvious reduction effect, and reach a broad market Prospect, high reactivity, effect of short curing time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
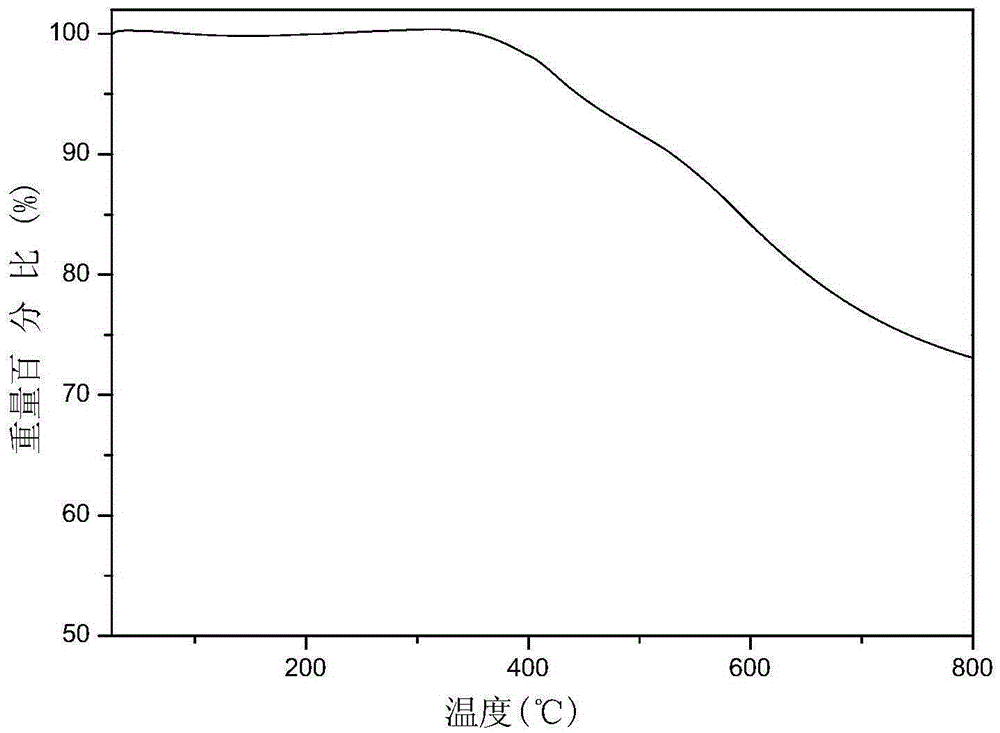
Image
Examples
Embodiment 1
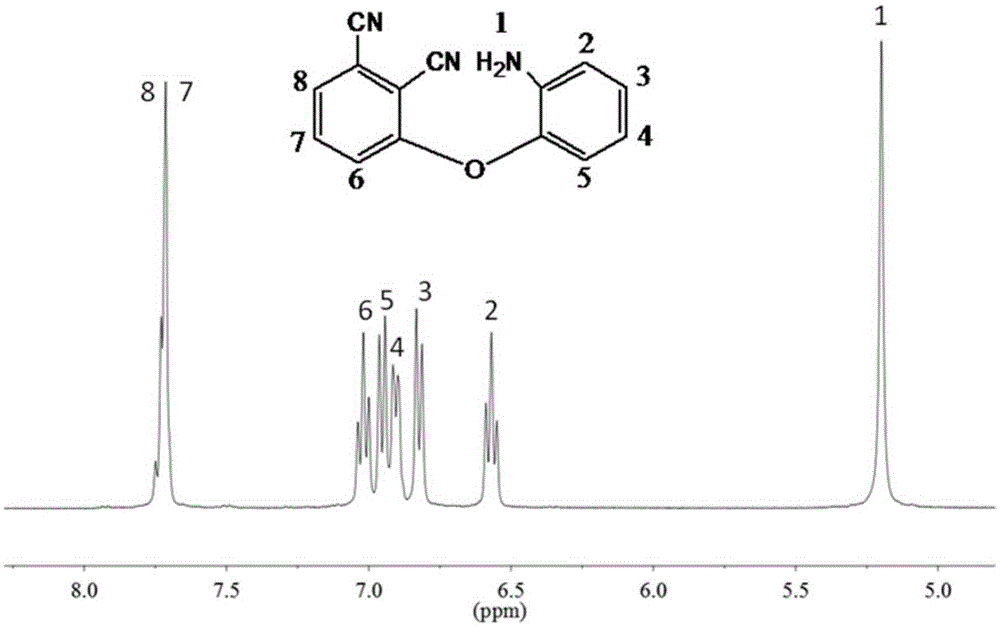
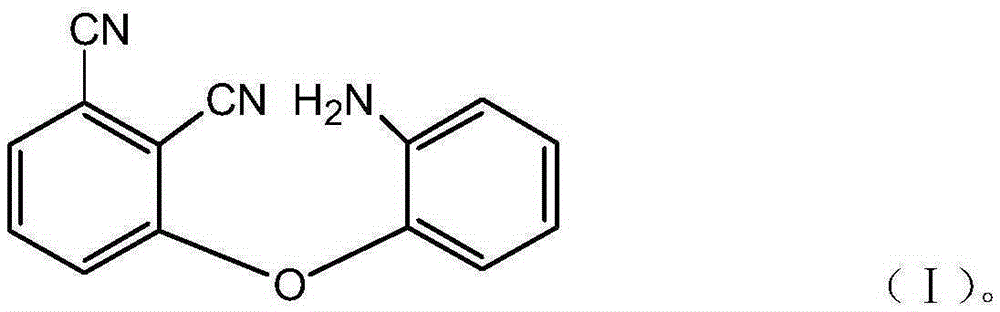
[0033] A kind of aminophenoxy phthalonitrile resin monomer of the present invention, molecular structural formula is following formula (I):
[0034]
[0035] A kind of synthetic method of the above-mentioned aminophenoxy phthalonitrile resin monomer of the present embodiment, comprises the following steps:
[0036] (1) Add 0.1mol of o-aminophenol, 0.15mol of anhydrous potassium carbonate, and 150ml of N,N-dimethylformamide DMF (solvent) into a dry reaction vessel (such as a three-necked flask), vacuumize and change nitrogen, and oil The bath was heated to 90°C for 1 hour.
[0037] (2) Add 0.08 mol of 3-nitrophthalonitrile to the above reaction vessel, reflux at 90° C. for 5 hours, and then cool to room temperature.
[0038] (3) Add 600ml of 0.1mol / L NaOH aqueous solution to the product solution obtained in step (2), a large amount of precipitation occurs, filter with suction, wash repeatedly with deionized water until neutral, filter, and place the resulting yellow solid p...
Embodiment 2
[0042] A kind of aminophenoxy phthalonitrile resin monomer of the present invention, molecular structure formula is identical with embodiment 1.
[0043] A method for synthesizing the aminophenoxyphthalonitrile resin monomer of this embodiment, the steps are basically the same as in Example 1, the only difference being that in step (1), the solvent used is dimethyl sulfoxide DMSO.
Embodiment 3
[0045] A kind of aminophenoxy phthalonitrile resin monomer of the present invention, molecular structure formula is identical with embodiment 1.
[0046] A method for synthesizing the aminophenoxyphthalonitrile resin monomer of this embodiment, the steps are basically the same as in Example 1, the only difference is that in step (2), the heating temperature is 85° C. and kept for 5 hours.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com