Selenourea warhead and building method thereof
A construction method and warhead technology, applied in peptide preparation methods, chemical instruments and methods, pharmaceutical formulations, etc., can solve problems such as slowness and weakening sirtuin inhibitory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
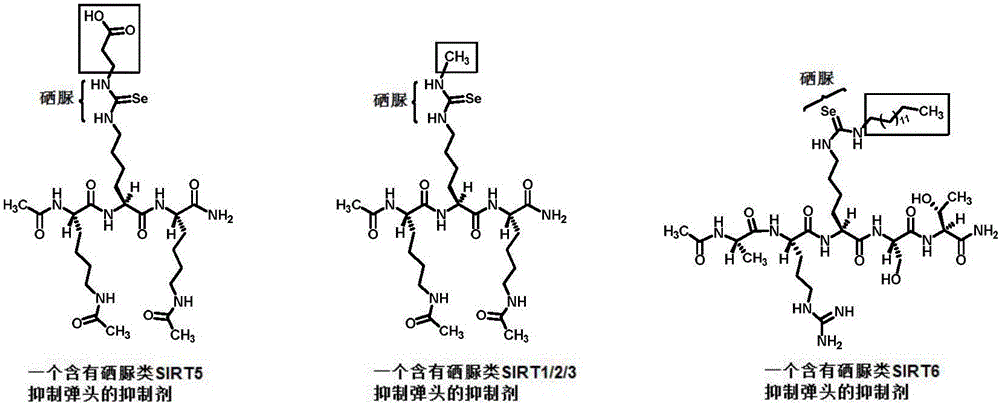
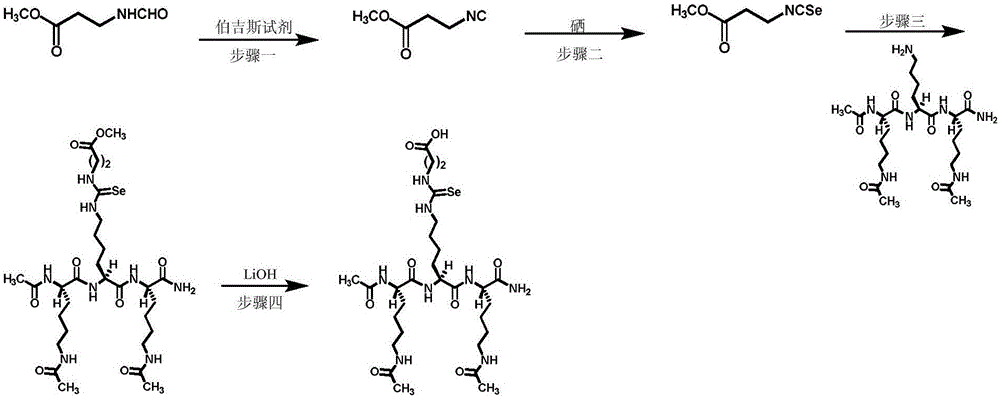
[0079] Embodiment 1. An inhibitor containing selenourea class SIRT5 inhibition warhead ( figure 1 ) chemical synthesis. The synthesis process of this compound is as follows figure 2 As shown, the specific steps are as follows:
[0080] Step 1. 3mmol H 3 COOC (CH 2 ) 2 Dissolve NHCHO in 15ml of DCM, add 3.6mmol of Burgess reagent, reflux for 2 hours to produce a yellow liquid; add 10ml of DCM to dilute, distill the water twice with 10ml, and discard the water layer; 5ml of saturated saline, keep organic layer. Anhydrous sodium sulfate was added to the organic layer to dry, filtered, and rotary evaporated to obtain product one.
[0081] Step 2. fully dissolve the product one in 30ml of tetrahydrofuran (THF), add triethylamine and 0.48g selenium powder and heat to reflux to fully react to generate a dark yellow liquid; use diatomaceous earth to filter the dark yellow liquid to obtain The filtrate, after being rotary evaporated and acidified with dilute hydrochloric acid, ...
Embodiment 2
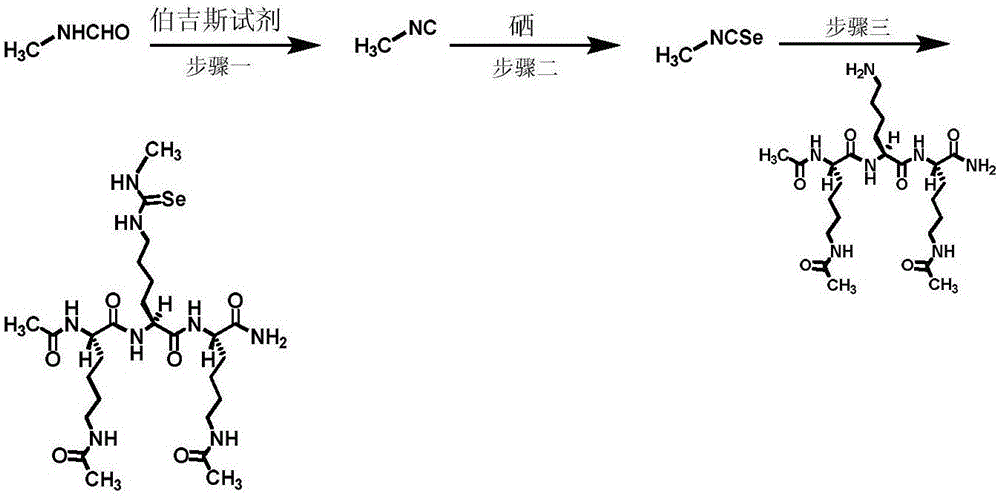
[0084] Example 2. An inhibitor containing selenourea SIRT1 / 2 / 3 inhibitor warhead ( figure 1 ) chemical synthesis. The synthesis process of this compound is as follows image 3 As shown, the specific steps are as follows:
[0085] Step 1. 2mmol CH 3 Dissolve NHCHO in 6ml of DCM, add 3mmol of Burgess reagent, reflux for 2h; add 5ml of DCM to dilute, distill water twice with 5ml, discard the water layer; 2ml of saturated saline, keep the organic layer. Anhydrous sodium sulfate was added to the organic layer to dry, filtered, and rotary evaporated to obtain product 1.
[0086] Step 2. Fully dissolve product one in 24ml of tetrahydrofuran (THF), add triethylamine and 0.72g of selenium powder and heat to reflux for 5h to generate a crude product; use diatomaceous earth to filter the crude product to obtain the filtrate, which is spinned and diluted After acidifying with hydrochloric acid, extract twice with ethyl acetate to obtain an organic layer; continue to wash the organic l...
Embodiment 3
[0088] Embodiment 3. An inhibitor that contains selenourea class SIRT6 inhibition warhead ( figure 1 ) chemical synthesis. The synthesis process of this compound is as follows Figure 4 shown.
[0089] Step 1. By the method of solid-phase peptide synthesis (SPPS), the pentapeptide with fully protected side chains is attached to the MBHA resin.
[0090] Step 2. After being treated with 1% TFA / DMF solution, the lysine side chain at the middle position is selectively deprotected to release a free amino group.
[0091] Step 1a. 1 mmol H 3 C(CH 2 ) 11 Dissolve NHCHO in 10ml of DCM, add 1.6mmol of Burgess reagent, reflux for 3h; add 5ml of DCM to dilute, distill the aqueous solution twice with 15ml, and discard the aqueous layer; 10ml of saturated saline aqueous solution, keep the organic layer; in the organic layer Add anhydrous sodium sulfate to dry, filter, and rotary evaporate to obtain product one.
[0092] Step 1b. Fully dissolve product one in 15ml tetrahydrofuran (THF...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com