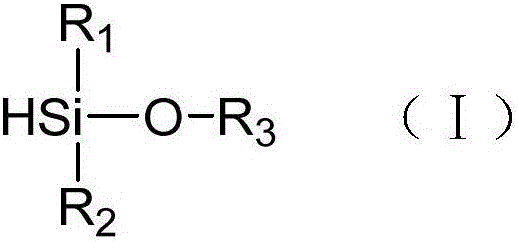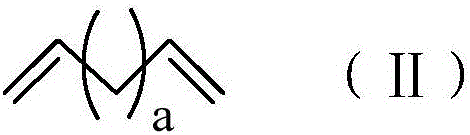Preparation method of alkoxy silane-olefin copolymer as well as product and application thereof
A technology of alkoxysilane and olefin copolymer, which is applied in the field of polyolefin functional modification, can solve problems such as low reactivity, unsatisfactory copolymerization effect, and difficulty in preparing metallocene catalysts, and achieve high reactivity and excellent crosslinking The effect of performance and easy structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
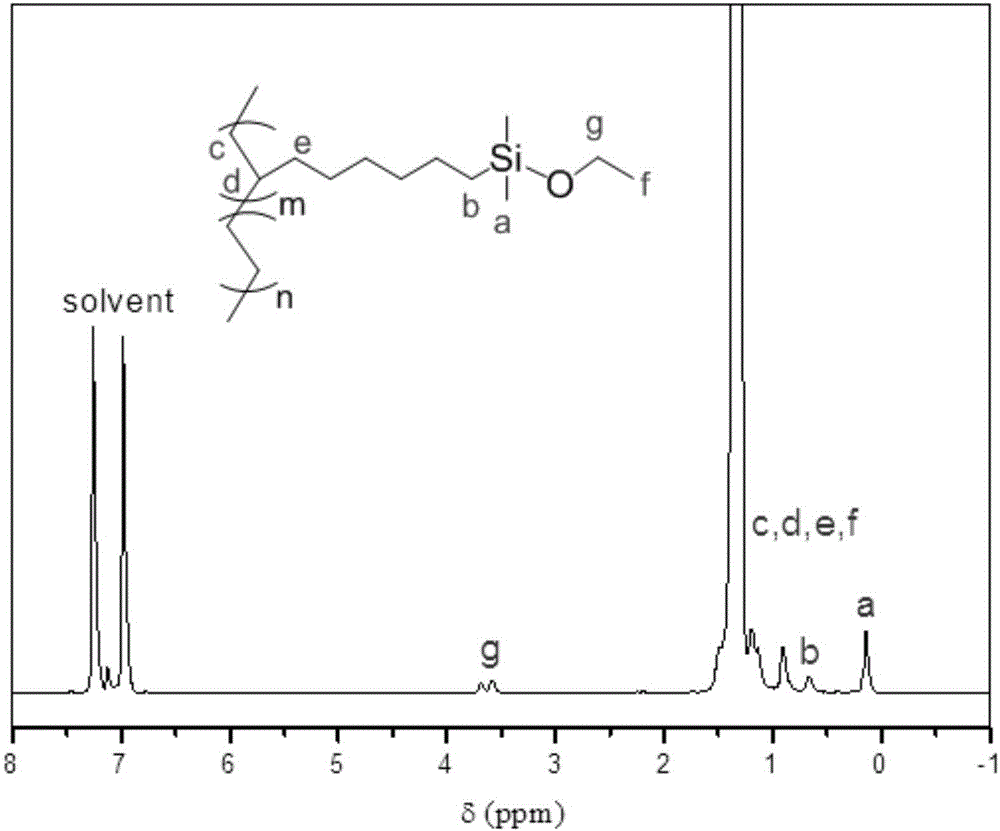
[0043] (1) In a 250mL three-necked flask, add 16.5g (150mmol) 1,7-octadiene, 30g toluene and 0.067g (30ppm) Karstedt catalyst solution (the mass concentration of platinum is 2%), and pass into the reaction system After nitrogen protection and stirring, 3.1 g (30 mmol) of dimethylethoxysilane was slowly added dropwise, and the reaction was continued at 40° C. for 8 hours after the dropwise addition was completed. After the reaction, the crude product is rotary evaporated to remove unreacted raw materials, and the catalyst is removed by passing through a silica gel column to obtain a clear and transparent oily liquid that is double bond-terminated alkoxysilane. It can be seen from the characterization of the hydrogen NMR spectrum that in the double bond-terminated alkoxysilane, a=4 and the purity is 98%.
[0044] (2) Fully dry the 250mL three-neck flask equipped with a mechanical stirring paddle to remove water and oxygen, continuously fill the system with ethylene gas and keep ...
Embodiment 2
[0048] Repeat the steps of Example 1, except that the amount of double bond-terminated alkoxysilane added in step (2) is 0.43g (20mmol / L), and the calculated reactivity is 5.8kg·mmol V -1 h -1 . The copolymerized product was confirmed to be a copolymer with alkoxysilane branch and olefin main chain by H NMR spectrum and infrared spectrum. It can be seen from the high temperature nuclear magnetic spectrum analysis that the molar content of the alkoxysilane component in the copolymer is 0.55%. As determined by high-temperature GPC analysis, the weight-average molecular weight of the copolymer is 29.7kg / mol, the PDI is 3.6, and the gel content of the product reaches 86% after crosslinking.
Embodiment 3
[0050] The procedure of Example 1 was repeated except that the monohydroterminated alkoxysilane added in step (1) was methyldiethoxysilane (4.0 g, 30 mmol). The product of step (1) is double bond-terminated alkoxysilane. It can be seen from the characterization of the hydrogen NMR spectrum that in the double bond-terminated alkoxysilane, a=4 and the purity is 98%.
[0051] In step (2), the amount of double bond-terminated alkoxysilane becomes 0.73g (30mmol / L), and the calculated reactivity is 3.8kg·mmol V -1 h -1 . The copolymerized product was confirmed to be a copolymer with alkoxysilane branch and olefin main chain by H NMR spectrum and infrared spectrum.
[0052] According to nuclear magnetic analysis, the molar content of the alkoxysilane component in the copolymer is 0.62%. According to high-temperature GPC analysis, the weight-average molecular weight of the copolymer is 18.4kg / mol, the PDI is 2.0, and the gel content of the product after crosslinking reaches 99%. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Weight average molecular weight | aaaaa | aaaaa |
| Weight average molecular weight | aaaaa | aaaaa |
| Weight average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com