Preparation method of 2-aryl benzoxazole and 2-aryl benzothiazole compounds
A technology of benzothiazole and benzoxazole, which is applied in the field of preparation of 2-aryl benzoxazole compounds, can solve complex processing problems and achieve the effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
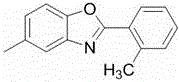
[0018] Example 1, 5-methyl-2-phenylbenzoxazole
[0019] Add 5mmol of 4-methyl-2-aminophenol 0.615g and 5 mmol of benzaldehyde 0.530g, 15ml of xylene into a 50ml reaction tube, stir at 120°C for 15 minutes, after the reaction is detected by TLC, add 1 mmol of imidazole Salt (4) 174mg, 1.25mmol potassium carbonate 175mg, reacted for 10 hours, the solution was spin-dried under reduced pressure, and then the obtained crude product was separated by column chromatography to obtain a product greater than 0.888 g, and the yield was greater than 85%.
[0020] Product structural formula (I)
[0021]
[0022] The characterization data are as follows:
[0023] 1 H NMR (400 MHz, CDCl 3 ) δ 8.24 (d, J = 3.6 Hz, 2H), 7.54 (d, J = 9.1 Hz,1H), 7.49 (s, 3H), 7.42 (d, J = 8.3 Hz, 1H), 7.12 (d, J = 8.2 Hz, 1H), 2.46(s, 3H); 13 C NMR (101 MHz, CDCl 3 ) δ 163.04, 148.96, 142.29, 134.30, 131.30,128.81, 127.50, 127.29, 126.17, 119.89, 109.89, 21.49. m / z (relative intensity, %...
Embodiment 2
[0024] Example 2, 2-(3-bromophenyl)-5-methyl-benzoxazole
[0025] Add 5mmol of 4-methyl-2-aminophenol 0.615g and 5 mmol of 3-bromobenzaldehyde 0.925g, 15ml of xylene into a 50ml reaction tube, stir at 120°C for 30 minutes, use TLC to detect the completion of the reaction, add 0.5mmol imidazolium salt (5) 109mg, 1.25mmol potassium carbonate 175mg, reacted for 15 hours, the solution was spin-dried under reduced pressure, and then the obtained crude product was separated by column chromatography to obtain a product greater than 1.31 g, and the yield was greater than 85%.
[0026] Product structure formula (II)
[0027]
[0028] The characterization data are as follows:
[0029] 1 H NMR (400 MHz, CDCl 3 ) δ 8.38 (t, J = 1.5 Hz, 1H), 8.15 (d, J = 7.8 Hz,1H), 7.63 (d, J = 8.0 Hz, 1H), 7.54 (s, 1H), 7.44 (d, J = 8.3 Hz, 1H), 7.37(t, J = 7.9 Hz, 1H), 7.17 (d, J = 8.3 Hz, 1H), 2.48 (s, 3H); 13 C NMR (101 MHz, CDCl 3 ) δ 161.48, 148.97, 142.05, 134.63, 134.19, 130.37...
Embodiment 3
[0030] Embodiment 3, 5-chloro-2-(methoxyphenyl)-benzoxazole
[0031]Add 5mmol of 4-chloro-2-aminophenol 0.615g and 5 mmol 2-methoxybenzaldehyde 0.680g, 15ml of xylene into a 50ml reaction tube, stir at 120°C for 10 minutes, and use TLC to detect the completion of the reaction. Add 109 mg of 0.5 mmol of imidazolium salt (5) and 175 mg of 1.25 mmol of potassium carbonate. After 12 hours of reaction, the solution is spin-dried under reduced pressure, and the obtained crude product is separated by column chromatography to obtain more than 1.10 g of the product, and the yield is more than 85%.
[0032] Product structure formula (III)
[0033]
[0034] The characterization data are as follows:
[0035] 1 H NMR (400 MHz, CDCl 3 ) δ 8.10 (d, J = 7.5 Hz, 1H), 7.78 (d, J = 1.0 Hz,1H), 7.52 – 7.45 (m, 2H), 7.28 (dd, J = 8.6, 1.5 Hz, 1H), 7.11 – 7.03 (m, 2H), 3.99 (s, 3H); 13 C NMR (101 MHz, CDCl 3 ) δ HRMS (ESI) Calcd for C 14 h 11 ClNO 2 : (M+H + ) 260.0478 (100.0%...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com