Preparation method of urokinase
A urokinase and cell technology, applied in the field of preparation of urokinase, can solve the problems of aglycosylation of urokinase, low efficiency of urokinase, difficult purification and the like, and achieve the effects of easy purification, high output and good thrombolytic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
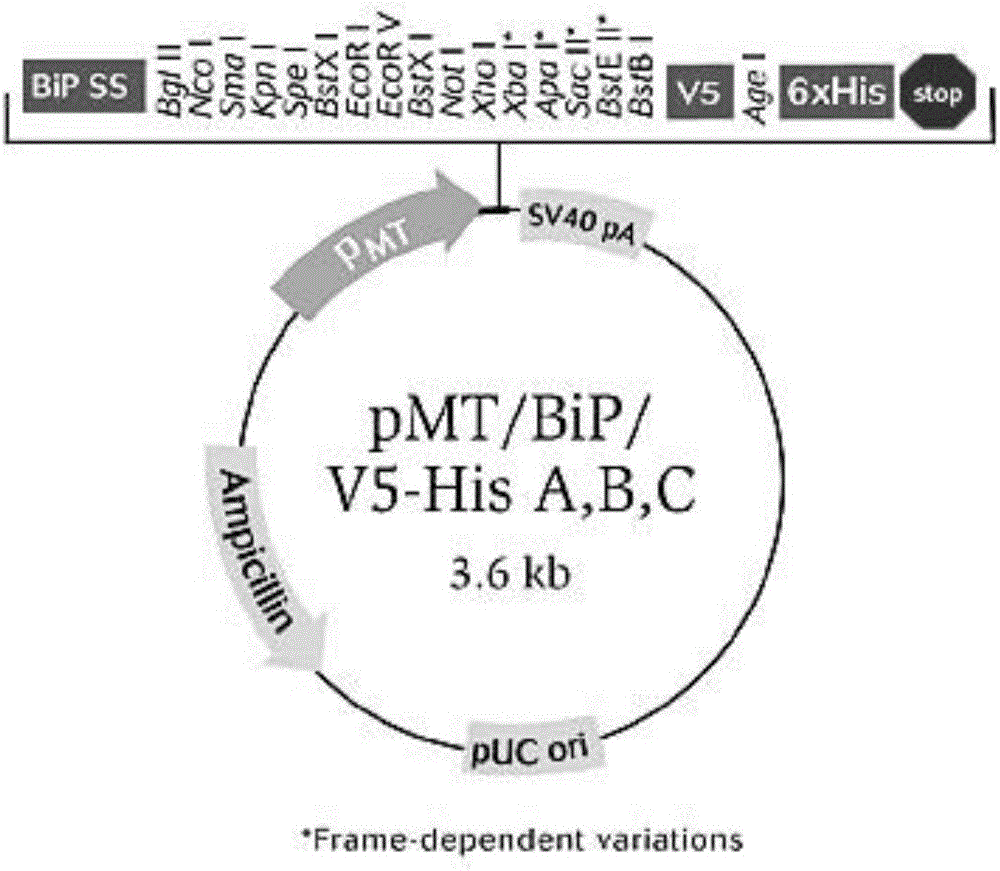
[0033] The human urokinase gene cDNA was cloned into the vector pMT / Bip / V5-A that can be expressed in Drosophila S2 cells with NcoI and XhoI sites, and 6 histidines were added at the end of the urokinase cDNA cDNA (CATCAT CAC CAT CAC CAT) for easy purification. The N-terminus of the plasmid contains Bip-signal sequence, so that the expressed urokinase can be released from cells for easy purification; it contains ampicillin and puromycin resistance genes, which is convenient for screening. Plasmid information such as figure 1 , figure 2 shown.
Embodiment 2
[0035] Transfect the plasmid obtained in Example 1 into Drosophila S2 cells, the specific operation is: place Drosophila S2 cells in 70-90% transfection mixture, dilute Reagent's transfection reagent culture medium (1:1 ratio dilution), dilute the plasmid in Medium. Add the diluted plasmid to the diluted 2000 reagent, and then incubated for 45min, the mixture was added to the cells.
[0036] 48 hours after transfection, selection was performed with Snyder's medium containing 2 μg / ml puromycin and 50 μg / ml ampicillin in 10% fetal bovine serum. Cells that were not successfully transfected were apoptotically lysed within 1-2 days in the culture medium, and cells that were successfully transfected could survive and be passaged in the culture medium. The medium was changed every four days, and after 3 weeks of selection, Drosophila S2 cells expressing urokinase were obtained.
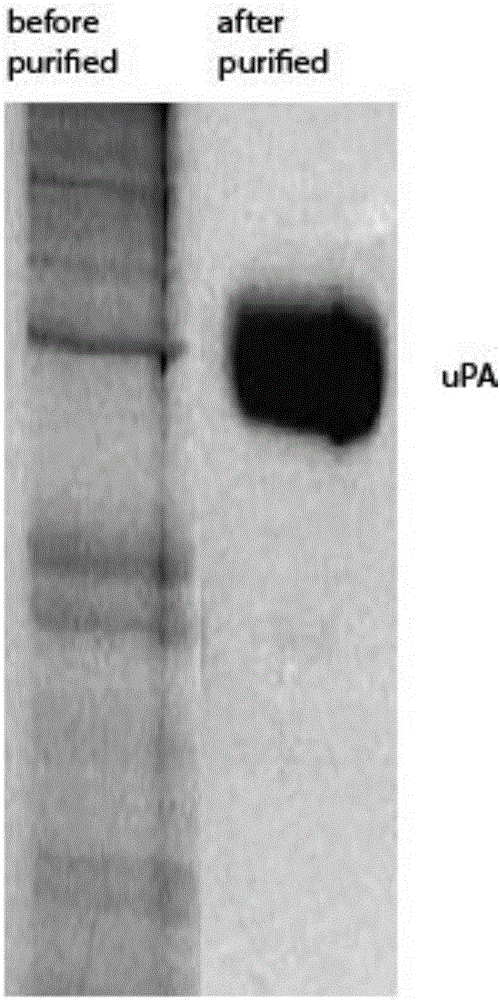
[0037] The Drosophila S2 cells that can express urokinase were subcultured and expanded. After o...
Embodiment 3
[0039] The interaction between uPA obtained in Example 2 and its receptor uPAR was analyzed by surface plasmon resonance (SPR) in BIAcore 2000 (BIAcore AB, Uppsala, Sweden). uPAR was immobilized on the carboxymethyldextran surface of a CM5 chip (BIAcore AB) using amine coupling according to the manufacturer's instructions. For the fixation procedure, uPAR was diluted to a final concentration of 2.5 μg / ml using sodium acetate buffer, pH 5.5. uPAR was immobilized on the surface for 7 min in separate flow cells at a flow rate of 10 μl / min, and a reference surface was prepared by amine coupling activation followed by immediate deactivation in the remaining two flow cells. For kinetic measurements, six different concentrations of uPA were injected for 3 minutes, followed by a 10-minute dissociation phase. At the end of each cycle, the surface was regenerated by a 1 min injection of 1 M NaCl followed by a 1 min injection of 0.02 M 6-aminocaproic acid, and a brief wash step with pho...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com