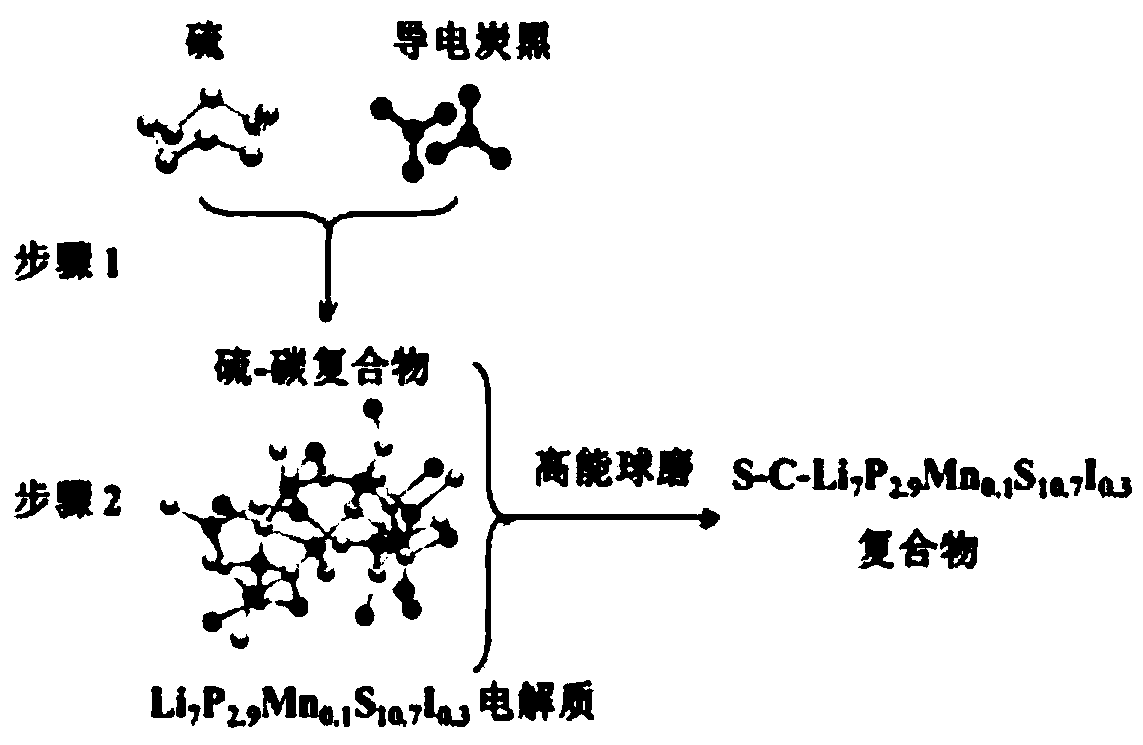
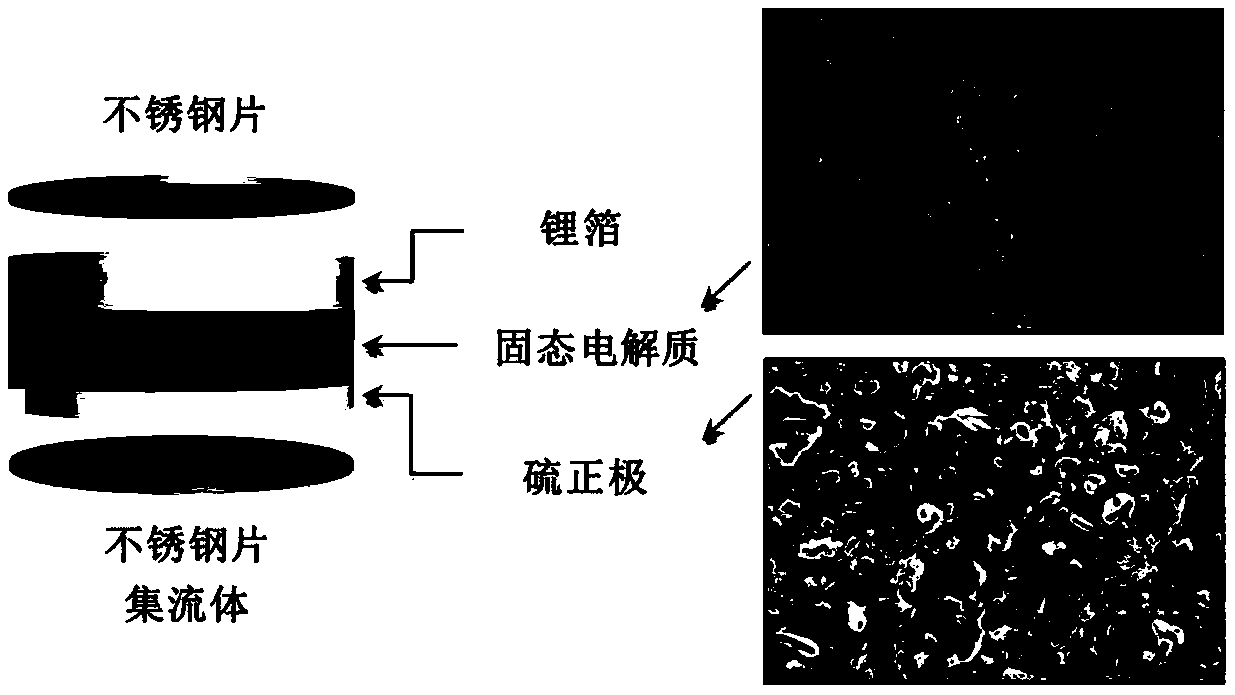
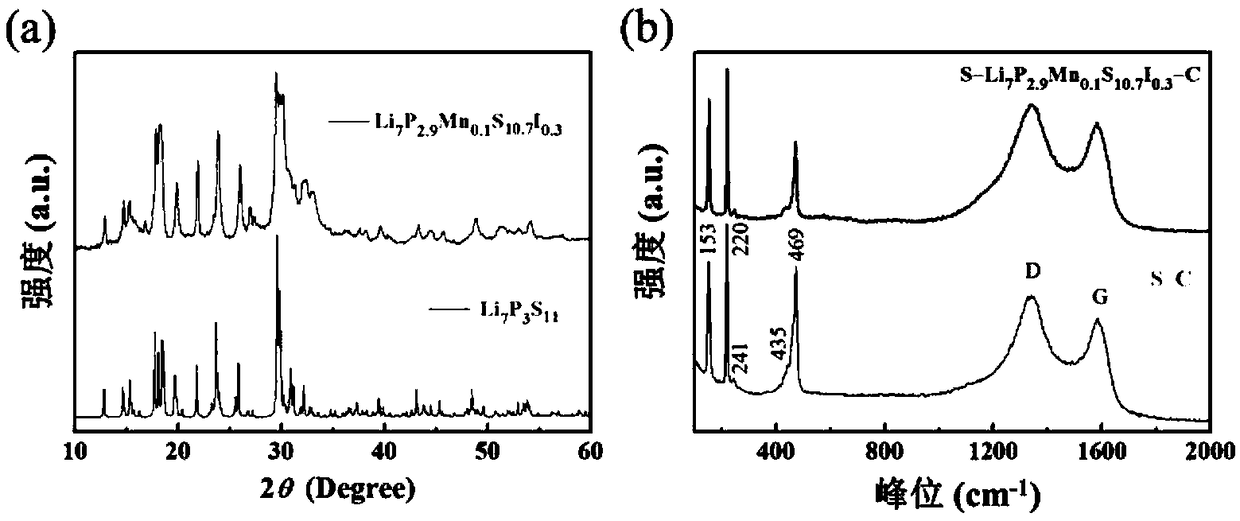
High ion conductivity sulfide solid electrolyte material and its preparation method and application
A solid electrolyte, ionic conductivity technology, applied in the direction of solid electrolyte, non-aqueous electrolyte, non-aqueous electrolyte battery, etc., can solve the problems of capacity decay, shortened cycle life, reduction of positive active material, etc. Low ionic conductivity, the effect of solving the shuttle effect and improving the cycle stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] (1) Will Li 2 S, P 2 S 5 Mixed with dopants MnS and LiI according to the molar ratio of 3.35:1.45:0.1:0.3 and high-energy mechanical ball milling, the speed and time of high-energy mechanical ball milling were 370rpm and 40 hours, so as to obtain the initial solid electrolyte material.
[0047] (2) The solid electrolyte raw material obtained in step (1) is placed in a tube furnace, and heat-treated under an argon atmosphere. The flow rate of argon gas for the reaction was 100 sccm, and the temperature and time of the reaction were 240° C. and 1 hour, respectively.
[0048] (3) Then, by grinding, the solid electrolyte obtained in step (2) is ground into powder, and the grinding is carried out under an inert atmosphere, the water content of the atmosphere is less than 1ppm, and the oxygen content is less than 1ppm. Then use a 200-450 mesh sieve to screen the solid electrolyte powder with a suitable particle size to obtain the MnS and LiI doped high ion conductivity sul...
Embodiment 2
[0053] (1) Will Li 2 S, P 2 S 5 Mixed with dopants MnS and LiI according to the molar ratio of 3.25:1.4:0.2:0.5 and high-energy mechanical ball milling, the speed and time of high-energy mechanical ball milling were 510rpm and 40 hours, so as to obtain the initial solid electrolyte material.
[0054] (2) The solid electrolyte raw material obtained in step (1) is placed in a tube furnace, and heat-treated under an argon atmosphere. The flow rate of argon gas for the reaction was 100 sccm, and the temperature and time of the reaction were 250° C. and 2 hours, respectively.
[0055] (3) Then, by grinding, the solid electrolyte obtained in step (2) is ground into powder, and the grinding is carried out under an inert atmosphere, the water content of the atmosphere is less than 1ppm, and the oxygen content is less than 1ppm. Then use a 200-450 mesh sieve to screen the solid electrolyte powder with a suitable particle size to obtain the MnS and LiI doped high ion conductivity sul...
Embodiment 3
[0059] (1) Will Li 2 S, P 2 S 5 Mixed with dopants MnS and LiI according to the molar ratio of 3.1:1.35:0.3:0.8 and high-energy mechanical ball milling, the speed and time of high-energy mechanical ball milling were 510rpm and 60 hours, so as to obtain the initial solid electrolyte material.
[0060] (2) The solid electrolyte raw material obtained in step (1) is placed in a tube furnace, and heat-treated under an argon atmosphere. The flow rate of argon gas for the reaction was 100 sccm, and the temperature and time of the reaction were 260° C. and 3 hours, respectively.
[0061] (3) Then, by grinding, the solid electrolyte obtained in step (2) is ground into powder, and the grinding is carried out under an inert atmosphere, the water content of the atmosphere is less than 1ppm, and the oxygen content is less than 1ppm. Then use a 200-450 mesh sieve to screen the solid electrolyte powder with a suitable particle size to obtain the MnS and LiI doped high ion conductivity sul...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com