Thiaxanthene dioxide type organic electroluminescence material and preparation method and application thereof
A technology of thiadioxide and luminescence, which is applied in the direction of luminescent materials, chemical instruments and methods, organic chemistry, etc., to achieve the effects of improving efficiency roll-off, reducing efficiency roll-off, and reducing device voltage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
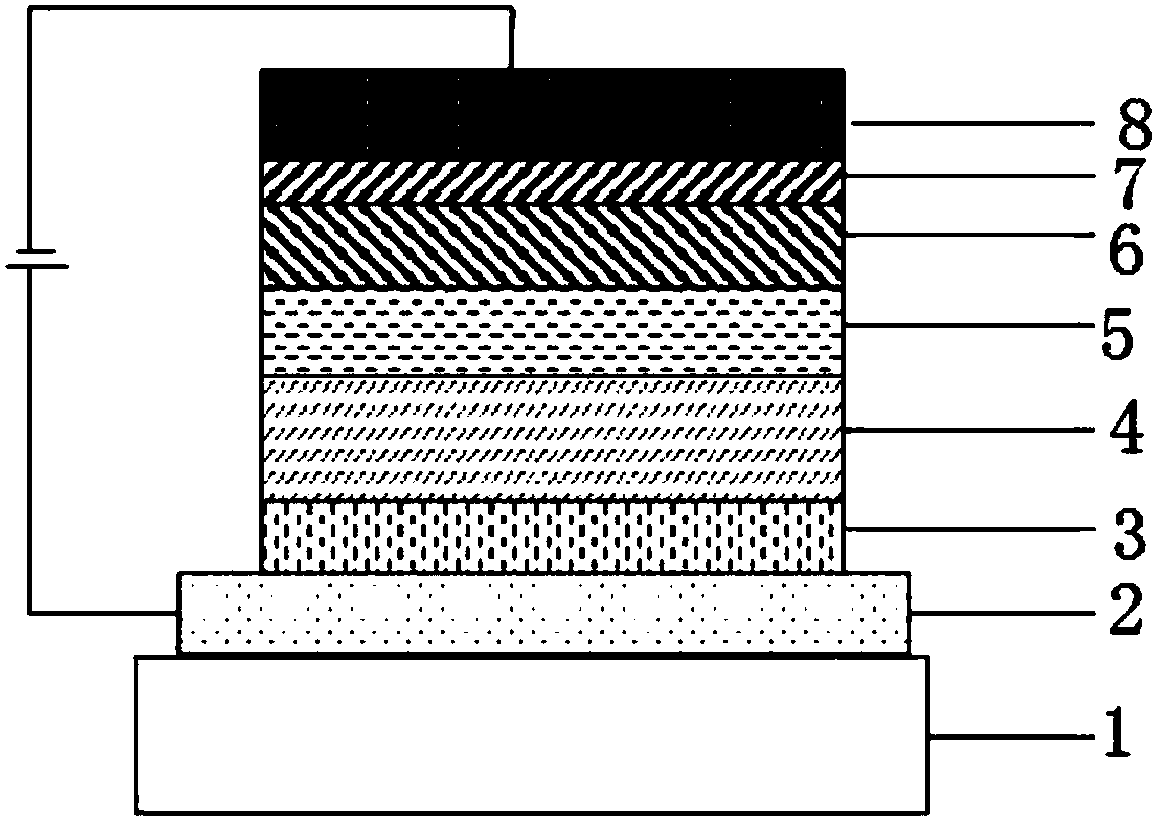
Image
Examples
Embodiment 1
[0047] Example 1 Compound A1
[0048]
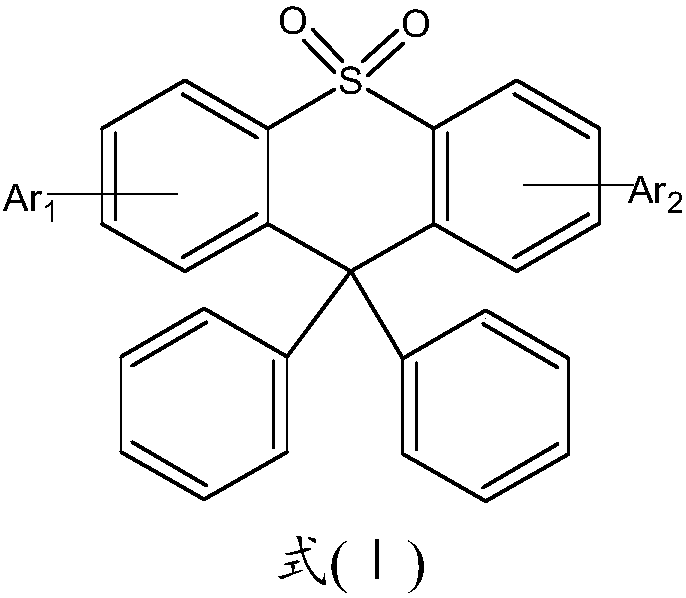
[0049] In a 250ml three-neck flask, under a nitrogen atmosphere, add 0.01mol 9,9-diphenyl-3-bromo-10,10-thioxanthene dioxide, 0.011mol compound C1, 0.03mol sodium tert-butoxide, 1× 10-4mol Pd 2 (dba) 3 , 1×10-4mol tri-tert-butylphosphine, 150ml toluene, heated and refluxed for 24 hours, sampled and spotted, cooled naturally, filtered, the filtrate was rotary evaporated, and passed through a silica gel column to obtain the target product with a purity of 98.56% and a yield of 54%.
[0050] High resolution mass spectrometry, ESI source, positive ion mode, molecular formula C 46 h 33 NSO 3 , theoretical value: 679.22, test value: 679.43.
Embodiment 2
[0051] Example 2 Compound A2
[0052]
[0053] In a 250ml three-neck flask, under a nitrogen atmosphere, add 0.01mol 9,9-diphenyl-3-bromo-10,10-thioxanthene dioxide, 0.011mol compound C2, 0.03mol sodium tert-butoxide, 1× 10-4mol Pd 2 (dba) 3 , 1×10-4mol tri-tert-butylphosphine, 150ml toluene, heated and refluxed for 24 hours, sampled and spotted, cooled naturally, filtered, the filtrate was rotary evaporated, and passed through a silica gel column to obtain the target product with a purity of 99.70% and a yield of 59%.
[0054] High resolution mass spectrometry, ESI source, positive ion mode, molecular formula C 46 h 33 NO 3 S, theoretical value: 679.22, test value: 679.58.
Embodiment 3
[0055] Example 3 Compound A3
[0056]
[0057] In a 250ml three-neck flask, under a nitrogen atmosphere, add 0.01mol 9,9-diphenyl-3-bromo-10,10-thioxanthene dioxide, 0.011mol compound C3, 0.03mol sodium tert-butoxide, 1× 10-4mol Pd 2 (dba) 3 , 1×10-4mol tri-tert-butylphosphine, 150ml toluene, heated to reflux for 24 hours, sampled and spot plated, cooled naturally, filtered, the filtrate was rotary evaporated, and passed through a silica gel column to obtain the target product with a purity of 99.80% and a yield of 46%.
[0058] High resolution mass spectrometry, ESI source, positive ion mode, molecular formula C 46 h 33 NO 3 S, theoretical value: 679.22, test value: 679.36.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com