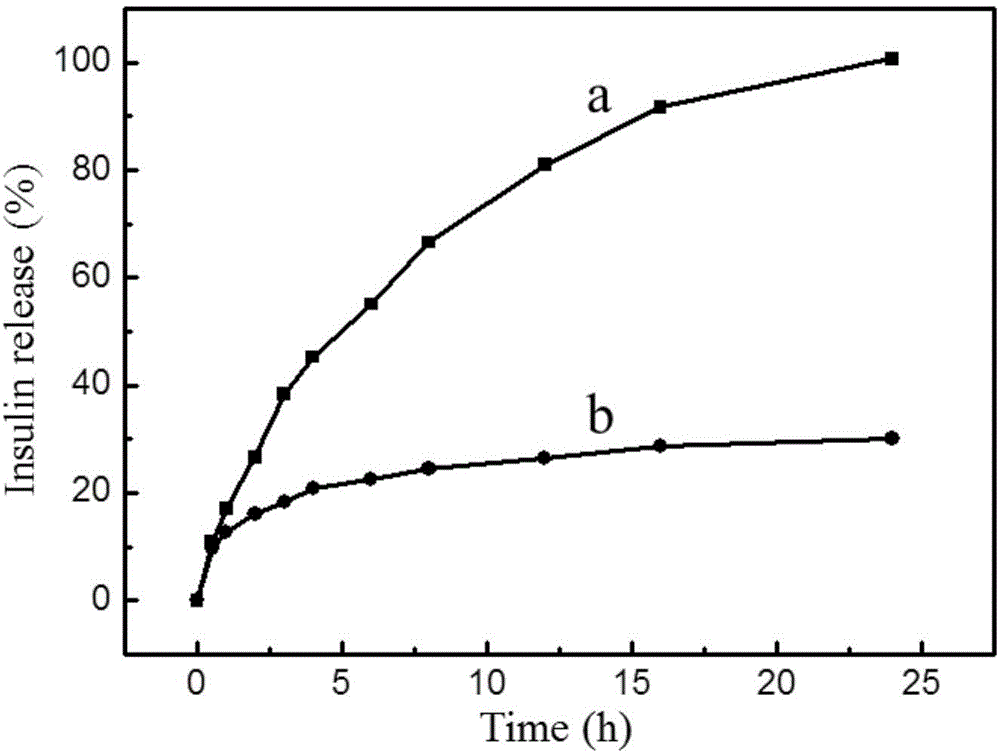
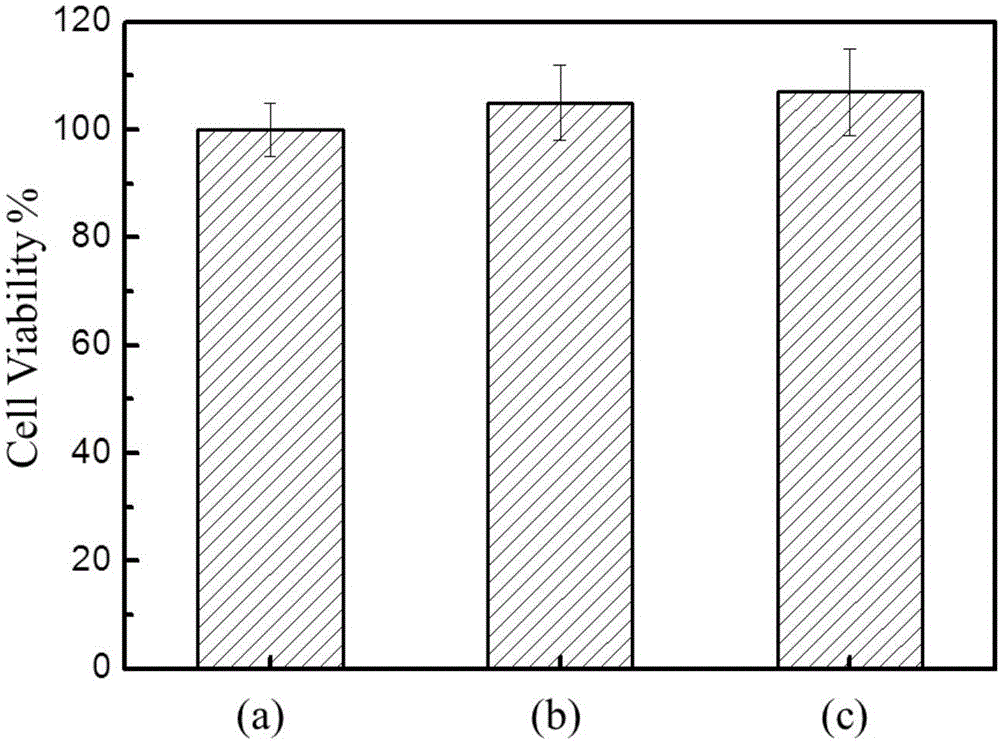
Preparation method of polydopamine microcapsule oral insulin administration carrier
A technology of polydopamine microcapsules and drug delivery carriers is applied in the directions of pharmaceutical formulations, medical preparations without active ingredients, and medical preparations containing active ingredients, etc. The effect of good capacitance and long residence time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1, a preparation method of a polydopamine microcapsule oral insulin delivery carrier, comprising the following steps:
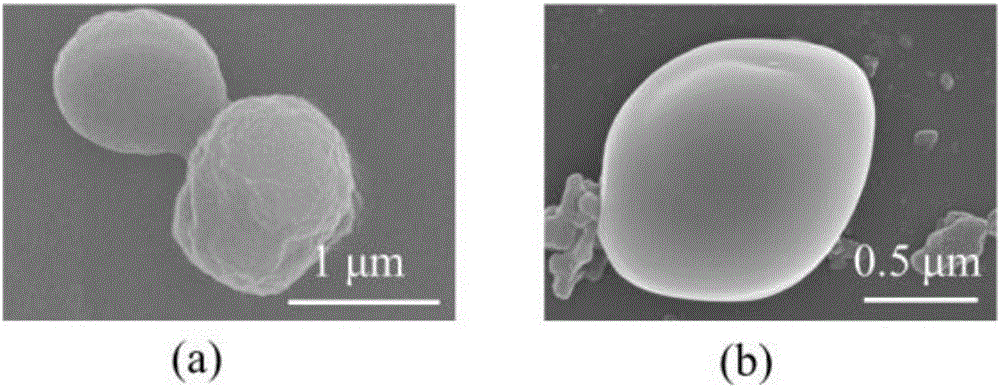
[0033] 1) Dissolve the insulin powder with a hydrochloric acid solution with a pH of 2 to a concentration of 10 mg / mL, add sodium chloride crystals thereinto until the amount of sodium chloride reaches 0.6 mol / L, and react for 1 h under shaking in a water bath at 15°C. It was observed that white insulin particles were gradually precipitated, and the product was centrifuged, and the product was washed twice with 2mol / L sodium chloride solution, and centrifuged to obtain insulin micro-nano particles;
[0034]2) First, prepare a Tris-HCl buffer solution with a pH of 7.5; secondly, disperse the insulin micronanoparticles in the Tris-HCl buffer solution so that the concentration of insulin is 10 mg / mL, add dopamine powder to make the concentration 2 mg / mL, The above reaction system was placed at room temperature and reacted at a speed of 900 rpm ...
Embodiment 2
[0043] Embodiment 2, a preparation method of a polydopamine microcapsule oral insulin delivery carrier, comprising the following steps:
[0044] 1) Dissolve the insulin powder with a hydrochloric acid solution with a pH of 1 to a concentration of 50 mg / mL; add sodium chloride crystals to it until the amount of sodium chloride reaches 0.5 mol / L; react for 3 hours under shaking in a water bath at 5°C, It was observed that white insulin particles were gradually precipitated; centrifuged, washed twice with 2mol / L sodium chloride solution, and centrifuged to obtain insulin micro-nano particles;
[0045] 2) Prepare a Tris-HCl buffer solution with a pH of 7.5, disperse the insulin micronanoparticles in the Tris-HCl buffer solution so that the concentration of insulin is 80 mg / mL, add dopamine powder to make the concentration 2 mg / mL; the above reaction system Place at room temperature and react at a speed of 900 rpm for 24 hours. During the reaction, the color of the solution gradual...
Embodiment 3
[0047] Embodiment 3, a preparation method of polydopamine microcapsule oral insulin delivery carrier, comprising the following steps:
[0048] 1) Use hydrochloric acid solution with a pH of 1.5 to dissolve the insulin powder to a concentration of 20 mg / mL; add sodium chloride crystals to it until the amount of sodium chloride reaches 2 mol / L; react for 1 hour under shaking in a water bath at 15°C, and observe The white insulin particles are gradually precipitated; centrifuged, washed twice with 2mol / L sodium chloride solution, and centrifuged to obtain insulin micro-nano particles;
[0049] 2) Prepare a Tris-HCl buffer solution with a pH of 8.5, disperse the upper insulin micro-nanoparticles in the Tris-HCl buffer solution so that the concentration of insulin is 40 mg / mL, add dopamine powder to make the concentration 2 mg / mL; The system was placed at room temperature and reacted at a speed of 900 rpm for 24 hours; during the reaction, the color of the solution gradually change...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com