Separation and determination method for 9-demethylation-alpha-dihydrotetrabenazine and impurities thereof
A technology of dihydrotetrabenazine and determination method, applied in the direction of material separation, measuring device, analysis material, etc., to achieve the effect of ensuring purity and labeling rate, benefiting effectiveness and safety, and sensitive detection method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
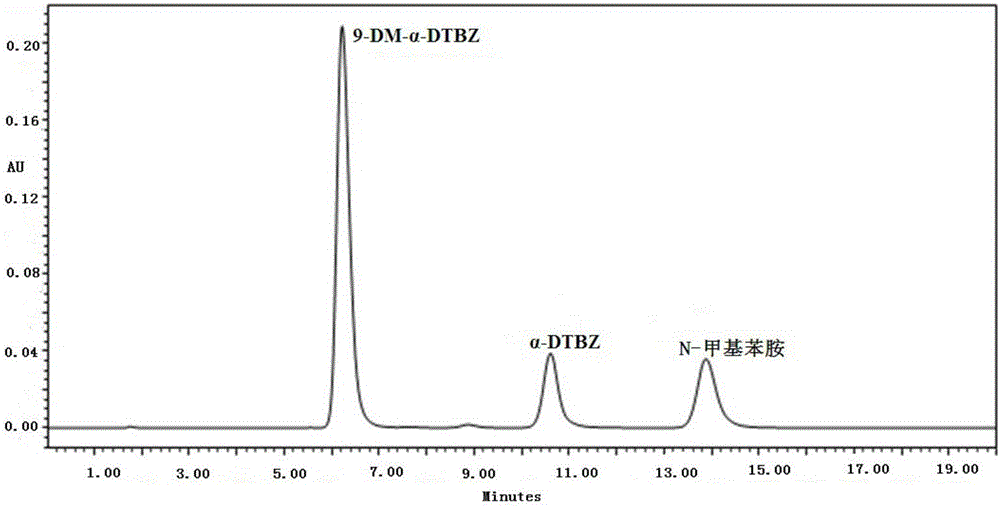
[0061] The separation and determination method of present embodiment 9-demethyl-alpha-dihydrotetrabenazine and impurities thereof comprises the following steps:
[0062] Weigh an appropriate amount of 9-desmethyl-α-dihydrotetrabenazine bulk drug to be tested, add ammonium acetate buffer solution (concentration is 50mM, pH value is 4.5)-methanol with a volume ratio of 70:30 to dissolve, and prepare Become the solution that concentration is 1mg / mL, as need testing solution;
[0063] Take an appropriate amount of reference substance of 9-desmethyl-α-dihydrotetrabenazine to make a solution with a concentration of 1 mg / mL as 9-desmethyl-α-dihydrotetrabenazine reference substance solution;
[0064] Take an appropriate amount of reference substance of α-dihydrotetrabenazine to make a solution with a concentration of 1 mg / mL as the α-dihydrotetrabenazine reference substance solution;
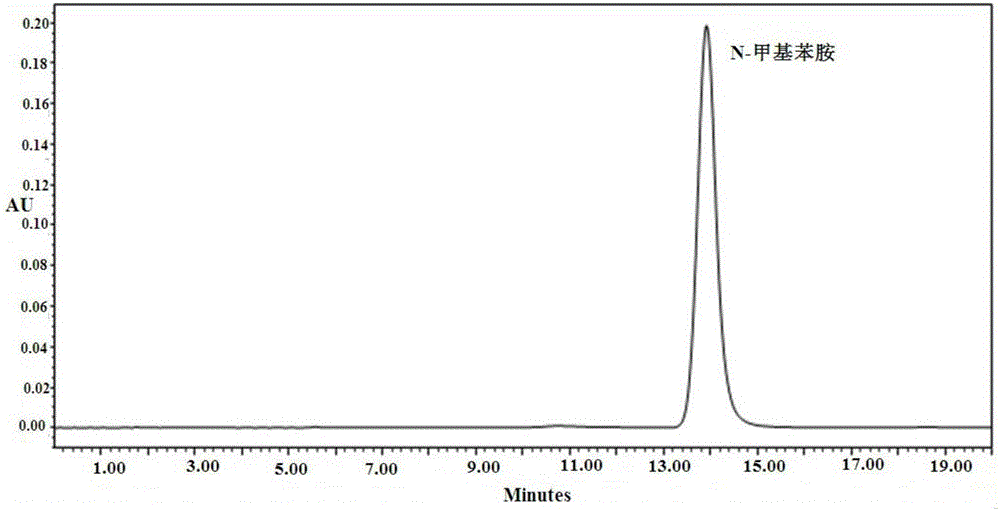
[0065] Take an appropriate amount of reference substance of N-methylaniline to make a solution with...
Embodiment 2
[0071] The separation and determination method of present embodiment 9-demethyl-alpha-dihydrotetrabenazine and impurities thereof comprises the following steps:
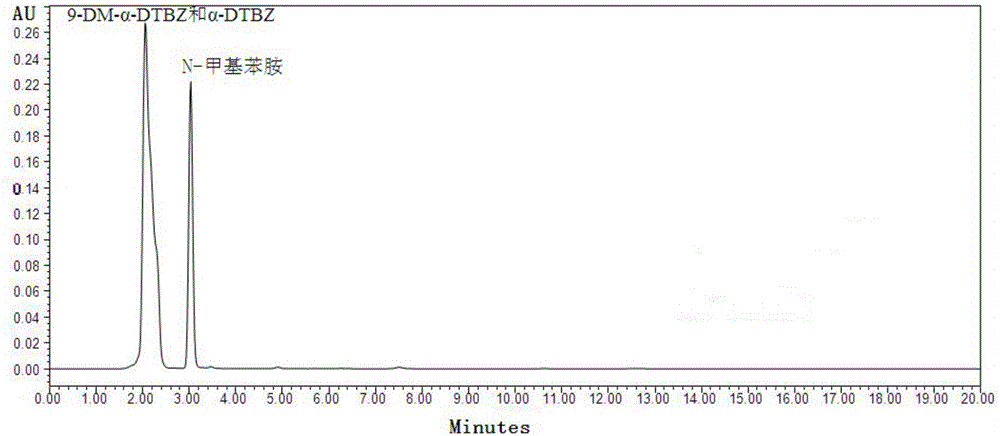
[0072] Weigh an appropriate amount of 9-desmethyl-α-dihydrotetrabenazine bulk drug to be tested, add ammonium acetate buffer solution (concentration is 50mM, pH value is 4.5)-methanol that the volume ratio is 50:50 and dissolve, prepare A solution with a concentration of 1.0 mg / mL was used as the test solution;
[0073] Take an appropriate amount of reference substance of 9-desmethyl-α-dihydrotetrabenazine to make a solution with a concentration of 1 mg / mL as 9-desmethyl-α-dihydrotetrabenazine reference substance solution;
[0074] Take an appropriate amount of reference substance of α-dihydrotetrabenazine to make a solution with a concentration of 1 mg / mL as the α-dihydrotetrabenazine reference substance solution;
[0075] Take an appropriate amount of reference substance of N-methylaniline to make a solution with ...
Embodiment 3
[0081] The separation and determination method of present embodiment 9-demethyl-alpha-dihydrotetrabenazine and impurities thereof comprises the following steps:
[0082] Weigh an appropriate amount of 9-desmethyl-α-dihydrotetrabenazine bulk drug to be tested, add ammonium acetate buffer solution (concentration is 50mM, pH value is 4.5)-methanol with a volume ratio of 75:25 to dissolve, and prepare Become the solution that concentration is 1mg / mL, as need testing solution;
[0083] Take an appropriate amount of reference substance of 9-desmethyl-α-dihydrotetrabenazine to make a solution with a concentration of 1 mg / mL as 9-desmethyl-α-dihydrotetrabenazine reference substance solution;
[0084] Take an appropriate amount of reference substance of α-dihydrotetrabenazine to make a solution with a concentration of 1 mg / mL as the α-dihydrotetrabenazine reference substance solution;
[0085] Take an appropriate amount of reference substance of N-methylaniline to make a solution with...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com