Catalyst, as well as preparation method and application thereof
A catalyst and carrier technology, applied in chemical instruments and methods, physical/chemical process catalysts, metal/metal oxide/metal hydroxide catalysts, etc., can solve problems such as carbon deposition, easy sintering, deactivation, etc., to achieve selective Good performance, good stability and high activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Preparation and Characterization of Catalyst Samples CAT-1~CAT-14 of Embodiment 1
[0061] Take a certain amount of active component metal salt and dissolve it in 10ml of water to make a solution, add 5g of hierarchical porous alumina, ultrasonic impregnation for a period of time, filter to remove the solvent and excess unabsorbed metal salt. The aluminum oxide adsorbed with metal ions was vacuum-dried at 80° C. for 8 hours, calcined in the air atmosphere, and finally reduced with hydrogen to obtain a multi-level porous supported multi-metal catalyst. If the above catalyst needs to be modified, the modified component precursor (such as alkali metal salt, alkaline earth metal salt or rare earth metal salt) can be made into a solution with the active component metal salt, and then impregnated, dried, roasted and reduction.
[0062] The relationship between the sample number and specific experimental parameters, iron series elements, noble metal elements, alkali metal ele...
Embodiment 2
[0068] Embodiment 2 Catalyst reaction evaluation
[0069] Take 0.2g of the catalyst sample CAT-1 and place it in a fixed-bed reactor with an inner diameter of 1cm. After hydrogen reduction is performed online, the temperature is adjusted to the reaction temperature. switch gas to CO 2 and CH 4 Mixed gas, N 2 is the internal standard. After the reaction, the gas is cooled and enters the gas chromatograph to detect the concentration of each substance, and calculate the CO 2 and CH 4 conversion rate.
[0070] Table 2
[0071]
[0072] CO 2 and CH 4 Conversion rates for are calculated using the following formulas:
[0073]
[0074]
[0075] where F CO2,in and F CO2,out is the CO in the feed gas and reaction tail gas 2 volume fraction flow; F CH4,in and F CH4,out CH in the reactants and products, respectively 4 volume fraction flow.
[0076] Under the same reaction conditions, the reaction results of catalyst samples CAT-2 to CAT-29 were similar to those of...
Embodiment 3
[0077] Embodiment 3 Catalyst stability evaluation
[0078] 0.2 g of catalyst samples CAT-1, CAT-2 and CAT-17 were respectively placed in a fixed-bed reactor with an inner diameter of 1 cm, and the catalyst stability was evaluated under the reaction condition A of Example 2.
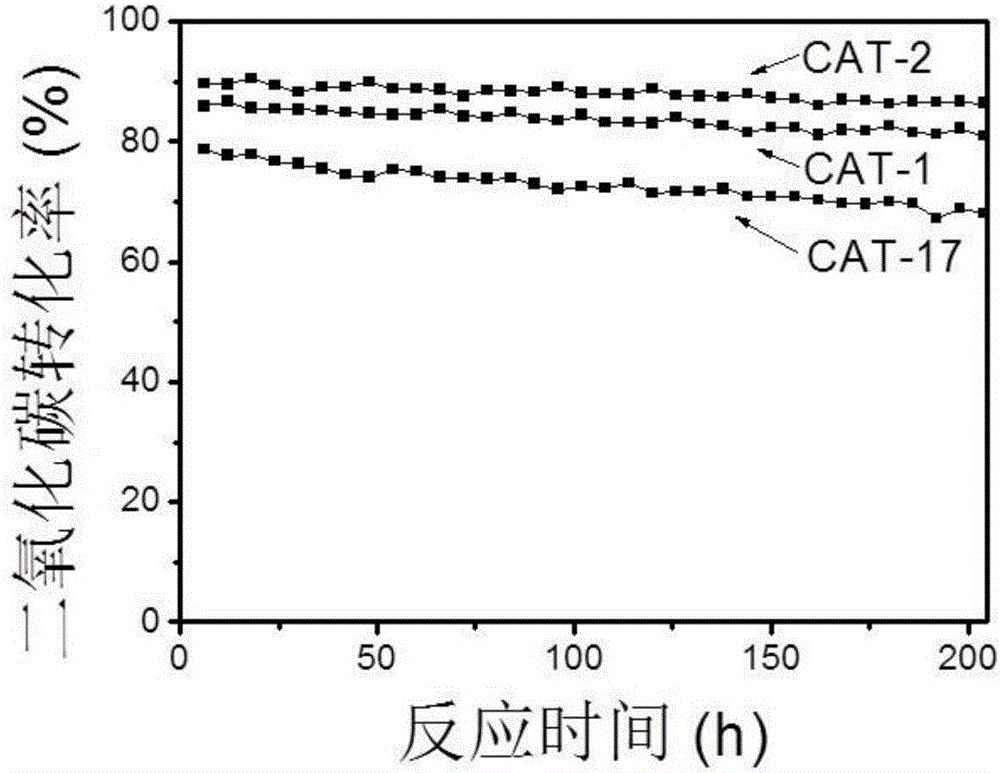
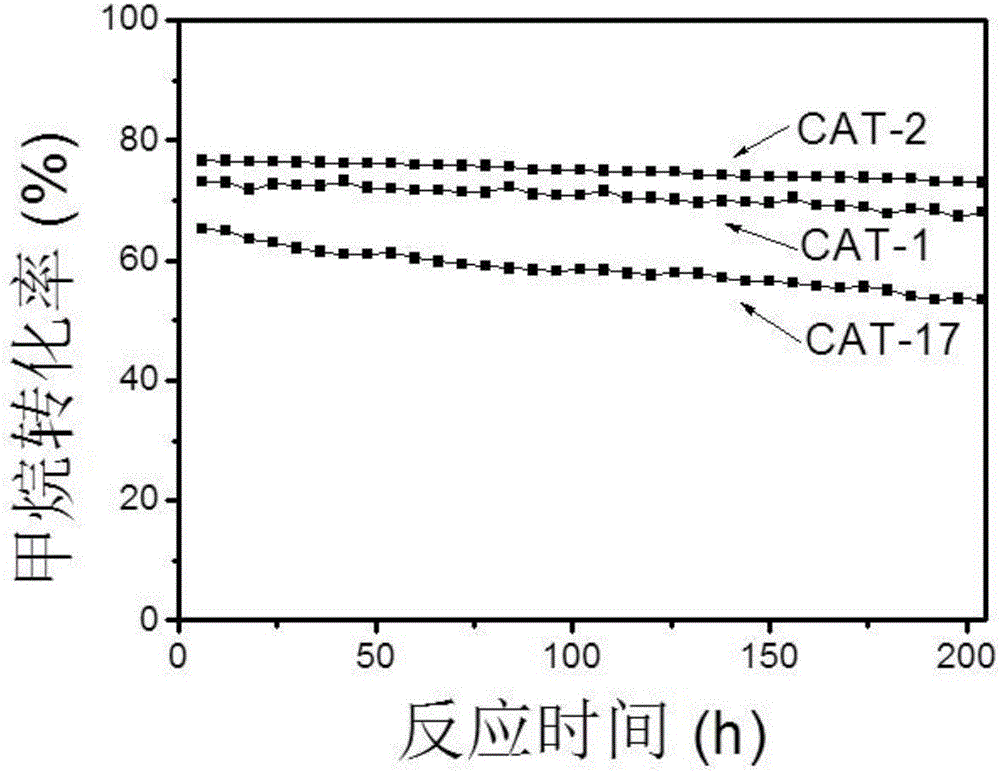
[0079]The stability test results of catalysts CAT-1, CAT-2 and CAT-17 are shown in Fig. 1(a) and Fig. 1(b). It can be seen from Figure 1 that the multimetal catalysts CAT-1 and CAT-2, which contain noble metals and iron-series elements in the active elements, under normal pressure and 800 ° C reaction conditions, within 204 hours of reaction time, the conversion of carbon dioxide and methane The rate maintains good stability. Active elements that do not contain noble metal elements have slightly lower catalyst stability. Catalyst stability: CAT-2>CAT-1>CAT-17, indicating that the addition of noble metals improves the stability of cobalt-based catalysts. At the same time, the data in Figure 1(a) and Fig...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com