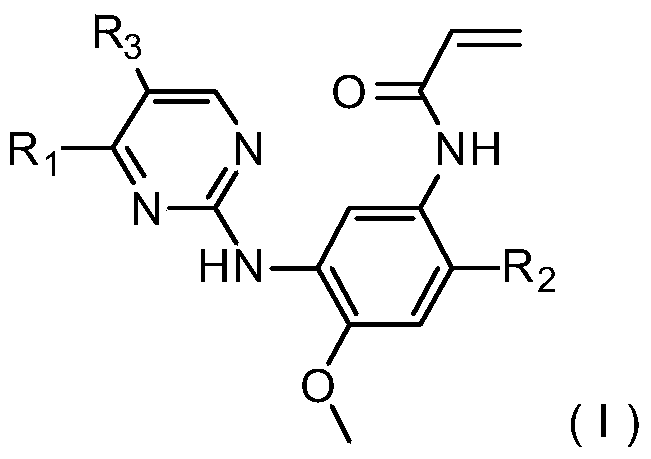
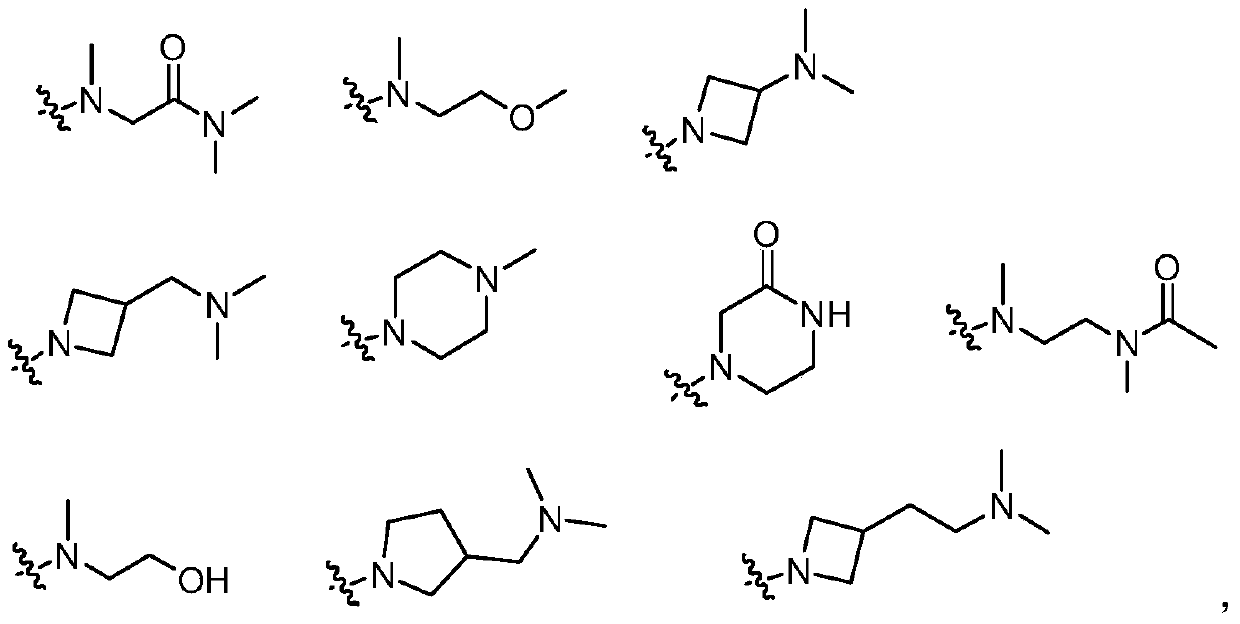
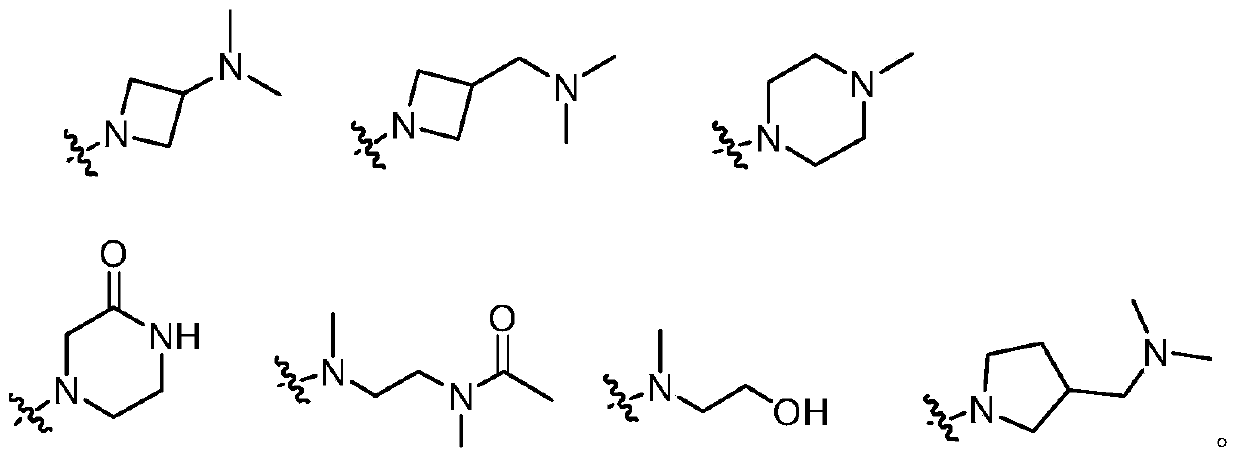
3-(4,5-substituted aminopyrimidine)phenyl derivatives and their applications
A drug and application technology, applied to 3-phenyl derivatives and its application field in the preparation of antitumor drugs, can solve the problems of low clinical tolerance of drugs, poor selectivity, and inability to solve clinical needs of drug resistance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0090]
[0091] The synthetic route is as follows:
[0092]
[0093] Compound FHND004‐03‐1
[0094] Take a 150mL sealed tube, add dimethylamine (40% in water) (8.87g, 78.76mmol), THF (80mL), and then 1-dibenzyl-3-methanesulfonic acid azetidine (25g) , 78.76mmol) refluxed and stirred for 8h, no raw material remained in TLC monitoring, and the solvent was distilled off under reduced pressure to obtain (FHND004-03-1) as a colorless liquid 10g.
[0095] Compound FHND004‐03‐2
[0096] Take a 250mL single-necked flask, add (FHND004‐03‐1) (10g, 37.54mmol), Pd / C (1.05g, 7.5mmol), MeOH (100mL), vacuum for 3 times hydrogen, stir at room temperature overnight, TLC monitoring No raw material remained, Pd / C was removed by filtration, and the solvent was distilled off under reduced pressure to obtain a yellow-green solid, which was purified by column chromatography, eluent (DCM:MeOH:NH) 3 H 2 O=10:1:0.1) eluted to obtain 3.5 g of a yellow liquid product (FHND004-03-2).
[0097] C...
Embodiment 2
[0105]
[0106] The synthetic route is as follows:
[0107]
[0108] Compound FHND004‐04‐1
[0109] Take a 50mL single-necked flask, respectively add TEA (3.99g, 39.47mmol), DCM (20mL), 1-dibenzyl-3-hydroxymethyl-azetidine (5g, 19.73mmol) and then MsCl (4.52 g, 39.47 mmol) was slowly added dropwise to the reaction solution at 0 °C, and continued to stir at room temperature for 2 h. TLC monitoring showed that no raw materials remained, and the solvent was distilled off under reduced pressure to obtain (FHND004‐04‐1) as a colorless liquid 8g .
[0110] Compound FHND004‐04‐2
[0111] Take 50mL sealed tube, add (FHND004‐04‐1) (8g, 24.14mmol), dimethylamine (40% in water) (10.88g, 96.55mmol), THF (10mL), reflux and stir for 6h, TLC monitoring is no longer The raw material remained, and the solvent was distilled off under reduced pressure to obtain a yellow-green liquid, which was purified by column chromatography, eluent (DCM:MeOH:NH) 3 H 2 O=10:1:0.1) eluted to obtain 6...
Embodiment 3
[0122]
[0123] The synthetic route is as follows:
[0124]
[0125] Compound FHND004‐05‐2
[0126] A 120 mL sealed tube was taken, and (FHND004-05-1) (2.0 g, 4.77 mmol), 1-methylpiperazine (477.16 mg, 4.77 mmol), DIPEA (0.92 g, 7.16 mmol), DMA (10 mL) were added. Then the tube was sealed at 140°C and reacted for 6h. TLC monitored no remaining raw materials. The reaction solution was cooled to room temperature, 20 mL of water was added, and the solid was precipitated, filtered, and then the filter cake was added to 2 mL of methanol to beat and wash, filter, and dry to obtain a red solid product ( FHND004‐05‐2) 1.9g.
[0127] Compound FHND004‐05‐3
[0128] Take a 50mL single-necked flask, add (FHND004‐05‐2) (1.9g, 3.80mmol), Pd / C (404.75mg, 0.38mmol), MeOH (20mL), vacuum for 3 times hydrogen, stir at room temperature overnight, TLC After monitoring that no raw material remained, Pd / C was removed by filtration, and the solvent was distilled off under reduced pressure to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com