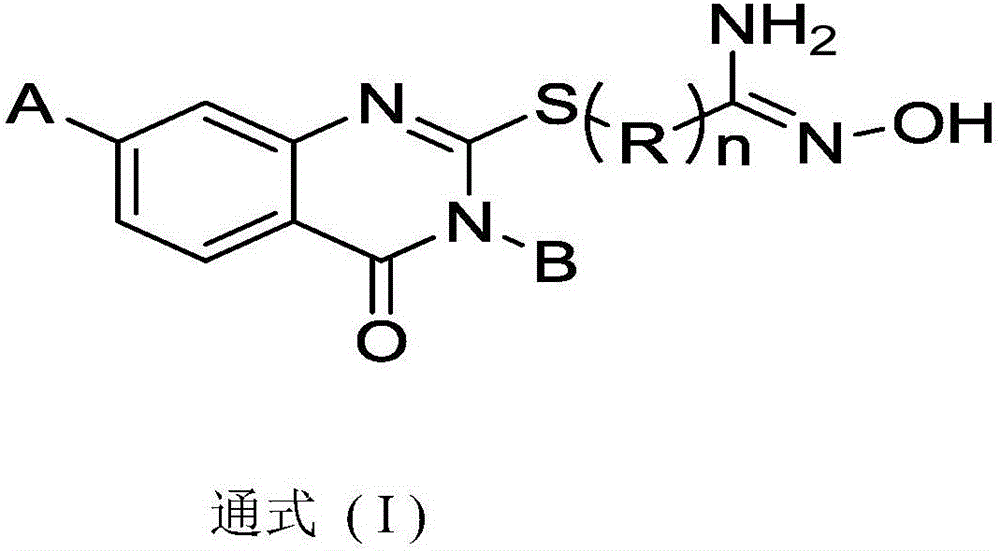
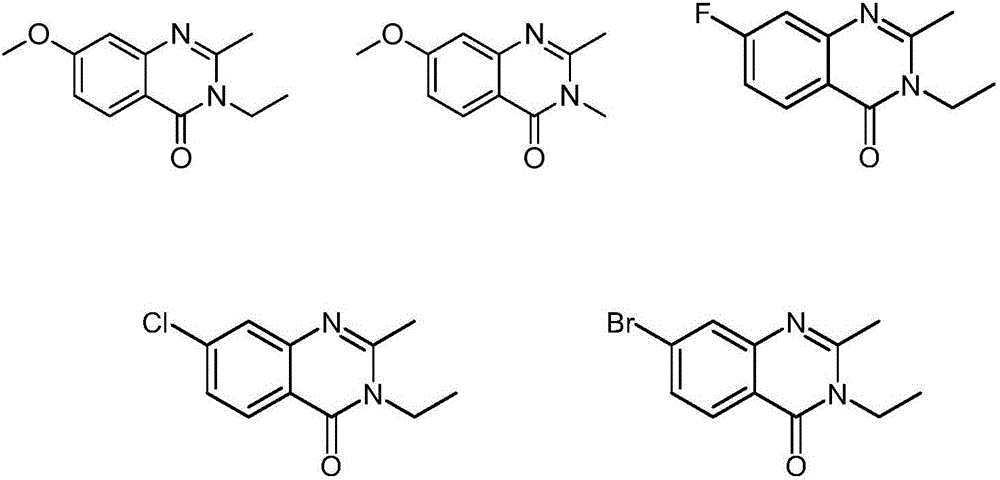
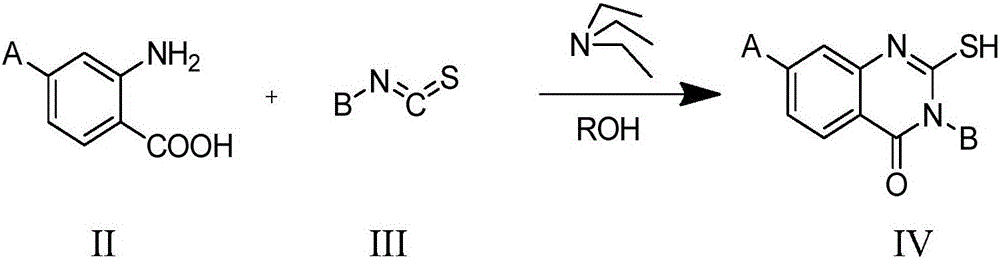
Amidoxime compound and application thereof to preparation of medicines for inhibiting cancer cell proliferation
A compound, amidoxime technology, applied in the field of amidoxime compounds and their application in the preparation of drugs for inhibiting cancer cell proliferation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Amidoxime Compound Example 1: [Compound 101]
[0044] (Z)-2-((3-Ethyl-7-methoxy-4-oxo-3,4-dihydro-quinazolin-2-yl)thio)-N'-hydroxyacetimide Amide
[0045]Step (1): Weigh 1.20g (7.2mmol) of 2-amino-4-methoxybenzoic acid, 0.63g (7.2mmol) of ethyl isothiocyanate, and 2.4ml of triethylamine in 100ml three-hole round bottom Add 35ml of ethanol to the flask, under nitrogen protection, magnetic stirring, and heat the oil bath until the ethanol refluxes. The reaction time is 2h. During this period, the reaction is followed by thin-layer chromatography. The developer is petroleum ether: ethyl acetate = 2:1. After the reaction is completed, cool to room temperature, vacuum filter the solid with a sand core funnel, wash the solid with ice ethanol, and then use petroleum ether to wash the solid and drain the solid. Finally a clean solid is obtained;
[0046] Step (2): Weigh 1g (4.23mmol) of the product obtained in the first step reaction, 0.66g (5.5mmol) of bromoacetonitrile, 1...
Embodiment 2
[0048] Amidoxime Example 2: [Compound 109]
[0049] (Z) 7-((3-ethyl-7-fluoro-4-oxo-3,4-dihydro-quinazolin-2-yl)sulfanyl)-N'-hydroxyheptanimide amide preparation.
[0050] Step (1): Weigh 1.55g (10mmol) of 2-amino-4-fluorobenzoic acid, 0.87g (10mmol) of ethyl isothiocyanate, and 3.10ml of triethylamine in a 100ml three-necked round-bottomed flask, and add 35ml of ethanol , nitrogen protection, magnetic stirring, the oil bath was heated to ethanol reflux, the reaction time was 2h, during which the reaction was followed by thin-layer chromatography, and the developer was petroleum ether: ethyl acetate = 2:1. After the reaction is completed, cool to room temperature, vacuum filter the solid with a sand core funnel, wash the solid with ice ethanol, and then use petroleum ether to wash the solid and drain the solid. Finally a clean solid is obtained;
[0051] Step (2): Weigh 1g (4.46mmol) of the product obtained in the first step reaction, 1.10g (5.80mmol) of bromoheptanitrile,...
Embodiment 3
[0053] Hydroxamic Acid Example 3: [Compound 113]
[0054] (Z) 7-((3-ethyl-7-fluoro-4-oxo-3,4-dihydro-quinazolin-2-yl)sulfanyl)-N'-hydroxyheptanimide amide preparation.
[0055] Step (1): Weigh 3.00g (17.50mmol) of 2-amino-4-chlorobenzoic acid, 1.50g (17.50mmol) of ethyl isothiocyanate, and 6.0ml of triethylamine in a 100ml three-necked round-bottomed flask, and add 35ml of ethanol, nitrogen protection, magnetic stirring, oil bath heating to ethanol reflux, the reaction time is 2h, during which the reaction is followed by thin-layer chromatography, the developer is petroleum ether: ethyl acetate = 2:1. After the reaction is completed, cool to room temperature, vacuum filter the solid with a sand core funnel, wash the solid with ice ethanol, and then use petroleum ether to wash the solid and drain the solid. Finally a clean solid is obtained;
[0056] Step (2): Weigh 1g (4.15mmol) of the product obtained in the first step of the reaction, 1.03g (5.40mmol) of bromoheptanitri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com