Genipin derivative and application thereof in preparing drug for preventing and treating neurodegenerative disease
A kind of methyl genipin and pharmaceutical technology, applied in the field of medicine, can solve the problems of poor blood-brain barrier permeability and bioavailability, limited pharmacological effects, low fat solubility, etc., achieve significant nerve cell protection activity, enhance cell Vitality, high fat-soluble effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
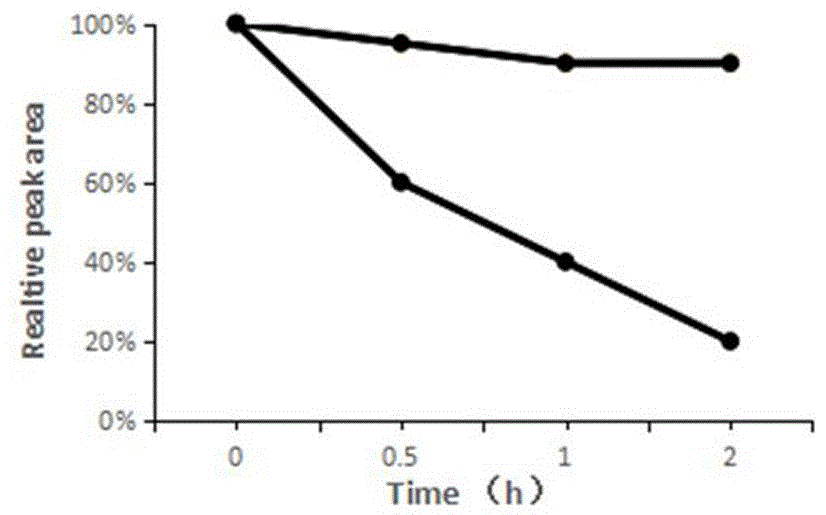
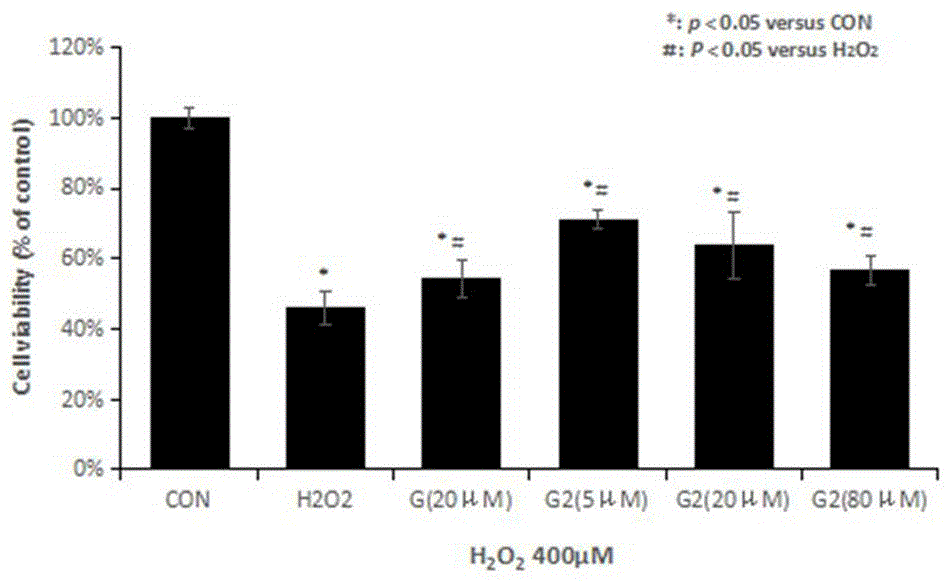
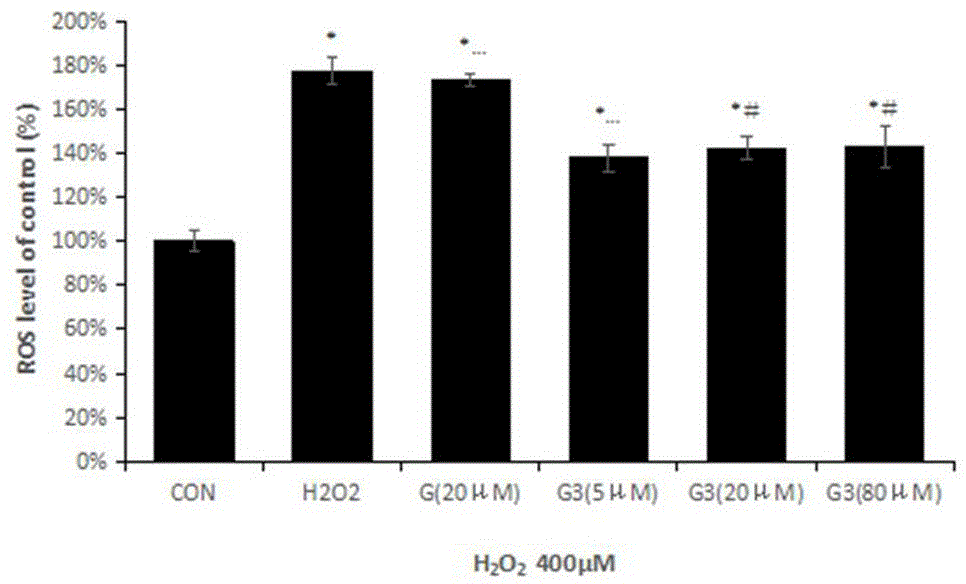
Image
Examples
Embodiment 1
[0049] Example 1: Preparation of compound G1. The genipin compound reacts with isopropanol under the catalysis of boron trifluoride ether to generate isopropoxygenipin. The obtained isopropoxygenipin (0.5mmol) was dissolved in 10ml of anhydrous dichloromethane, and the amount of benzoic acid equal to that of the substance was added, and after dissolving, 5mg of 4-dimethylaminopyridine (DMAP) was added, and 920mg of bicyclic Hexylcarbodiimide (DCC), stirred at room temperature, monitored by TLC. After the reaction was completed, it was filtered, separated by silica gel column chromatography (eluted with petroleum ether-ethyl acetate), and the solvent was removed under reduced pressure to obtain compound G1. Molecular formula(MW):C 21 h 24 o 6 (372.41g / mol); white oil; 63.0% Yield; 1 H NMR (600MHz, CDCl 3 )δ1.22(3H,d,J=6.14Hz),1.29(3H,d,J=6.21Hz),2.10(1H,m),2.66(1H,t,J=8.25Hz),2.92(1H, dd,J=16.57,8.56Hz),3.24(1H,q,J=8.36Hz),3.73(3H,s),4.06(1H,m),4.66(1H,d,J=8.35Hz),4.92( ...
Embodiment 2
[0054] Embodiment 2: Compound G2, Molecular formula (MW): C 22 h 26 o 6 (386.44g / mol); lightwhite oil; 72.0% Yield; 1 H NMR (600MHz, DMSO-d 6 )δ1.01(3H,d,J=6.09Hz),1.05(3H,d,J=6.18Hz),2.15(1H,m),2.52(3H,s),2.67(1H,dd,J=15.24 ,6.66Hz),3.02(2H,m),3.64(3H,s),3.84(1H,m),4.87(2H,q,J=7.56Hz),5.39(1H,d,J=3.06Hz), 5.93(1H,s),7.32(2H,m),7.44(1H,d,J=1.07Hz),7.49(1H,td,J=7.66,1.25Hz),7.87(1H,d,J=7.81Hz ); 13 C-NMR (150MHz, DMSO-d 6 )δ21.01,21.26,23.05,33.69,38.42,45.73,50.90,62.14,70.34,96.66,110.68,125.92,129.36,130.07,131.32,131.55,132.10,136.81,139.07,151.17,166.56,166.92;ESI-MS (m / z):387.1([M+H] + ).
Embodiment 3
[0055] Embodiment 3: Compound G3, Molecular formula (MW): C 22 h 26 o 6 (386.44g / mol); lightwhite oil; 70.0% Yield; 1 H NMR (600MHz, CDCl 3 )δ1.14(3H,d,J=6.11Hz),1.18(3H,d,J=6.19Hz),2.10(1H,m),2.39(3H,s),2.62(1H,t,J=8.18 Hz), 2.76(1H,dd,J=16.01,8.15Hz),3.08(1H,q,J=8.54Hz),3.65(3H,s),4.02(1H,m),4.80(1H,d,J =8.09Hz), 4.85(1H,d,J=12.74Hz),4.99(1H,d,J=14.25Hz),5.93(1H,s),7.34(2H,d,J=8.02Hz),7.52( 1H, d, J = 0.74Hz), 7.90 (2H, d, J = 8.17Hz); 13 C-NMR (150MHz, CDCl 3)δ21.08, 21.47, 23.23, 35.34, 38.36, 45.90, 50.94, 62.42, 71.52, 99.55, 110.06, 126.85, 129.19, 129.25, 138.36, 143.62, 152.19, 165.26, 166.86 387.1([M+H] + ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com