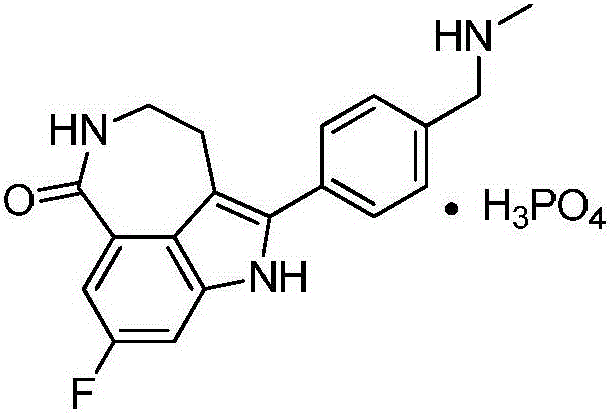
Synthesis process for Rucaparib intermediate of ovarian cancer treating medicine
A synthesis process and intermediate technology, which is applied in the field of synthesis technology of ovarian cancer drug intermediates, can solve the problems of few reaction steps, many reaction steps, long reaction time and the like, and achieves few reaction steps, short reaction time and low production cost. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Synthesis of 3-(2-bromoethyl)-7-fluoro-indole-5-carboxylic acid methyl ester
[0026] Under nitrogen protection, 19.3g (100mmol) of methyl 7-fluoro-indole-5-carboxylate, 0.97g of palladium tetrakistriphenylphosphine, 41.4g (300mmol) of potassium carbonate and 20.7g of 1,2-dibromoethane (110mmol) was refluxed in 150ml of acetonitrile for 2 hours to monitor the end of the reaction. The reaction solution was cooled to room temperature, filtered, the filtrate was washed with water, extracted three times with ethyl acetate, the organic phases were combined, dried over anhydrous sodium sulfate, concentrated under reduced pressure, and the Crystallization gave 27.5 g of methyl 3-(2-bromoethyl)-7-fluoro-indole-5-carboxylate, with a yield of 91.7%.
[0027] 1 H NMR (400MHz, CDCl 3 )δ: 2.40 (t, 2H) 3.51 (t, 2H) 3.64 (s, 3H) 7.11 ~ 7.18 (m, 2H) 7.44 (m, 1H) 11.22 (s, 1H).
Embodiment 2
[0029] Synthesis of 3-(2-bromoethyl)-7-fluoro-indole-5-carboxylic acid methyl ester
[0030] Under nitrogen protection, 38.6g (200mmol) of methyl 7-fluoro-indole-5-carboxylate, 3.8g (10%) of palladium tetrakistriphenylphosphine, 130.3g (400mmol) of cesium carbonate and 1,2-dibromo 22.6g (120mmol) of ethane was refluxed in 200ml of acetonitrile for 2 hours. After monitoring the end of the reaction, the reaction liquid was cooled to room temperature, filtered, the filtrate was washed with water, and extracted three times with ethyl acetate. The organic phases were combined, dried over anhydrous sodium sulfate, and concentrated under reduced pressure. , recrystallized from petroleum ether to obtain 56.2 g of methyl 3-(2-bromoethyl)-7-fluoro-indole-5-carboxylate, with a yield of 93.6%.
Embodiment 3
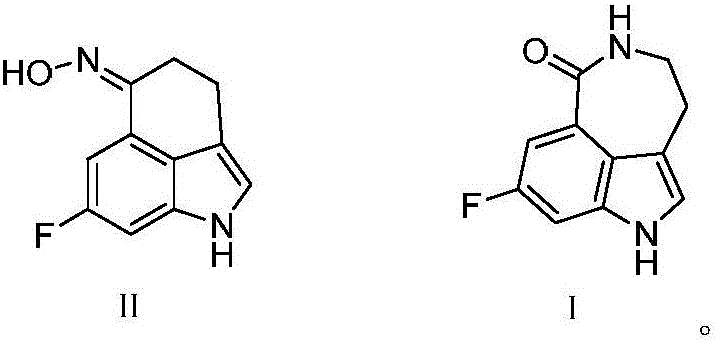
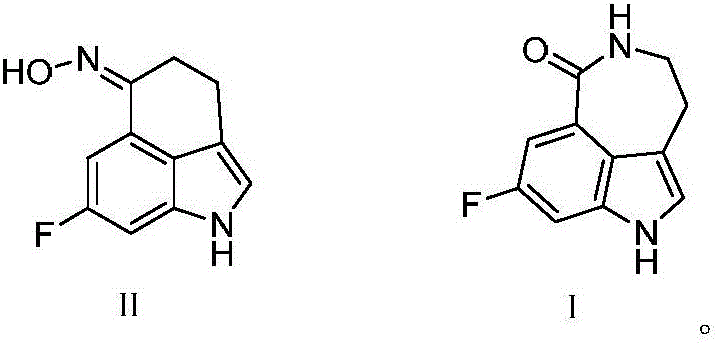
[0034] The synthesis of the compound shown in formula II:
[0035]Under nitrogen protection, 6 g (20 mmol) of 3-(2-bromoethyl)-7-fluoro-indole-5-carboxylic acid methyl ester obtained in step 1), 0.2 g iodine and 0.5 g (21 mmol) of dry magnesium chips were added Stir in 120ml of absolute diethyl ether for 1.5 hours until 3-(2-ethylmagnesium bromide)-7-fluoro-indole-5-carboxylic acid methyl ester is generated, then adjust the temperature of the resulting reaction solution to 45°C and stir for 3 hours , monitored until the end of the reaction, cooled in an ice bath and poured into saturated ammonium chloride, extracted with dichloromethane, washed, concentrated and dried to obtain 8-fluoro-cyclohexyl[5,4,3-cd]indol-6-one 3.44 g.
[0036] 3.78g (20mmol) of 8-fluoro-cyclohexyl[5,4,3-cd]indol-6-one, 21.7g (24mmol) of hydroxylamine hydrochloride and 0.73g (4mmol) of copper acetate were added to ethanol, and then Aqueous ammonia was added dropwise under stirring to adjust the pH to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com