Mixed-valence hexavanadate alkoxyl derivative and preparation method thereof
A technology of hexavanadate and mixed valence state, which is applied in the direction of chemical instruments and methods, compounds of group 5/15 elements of the periodic table, organic compounds of group 5/15 without C-metal bonds, etc., to achieve structurally rich Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
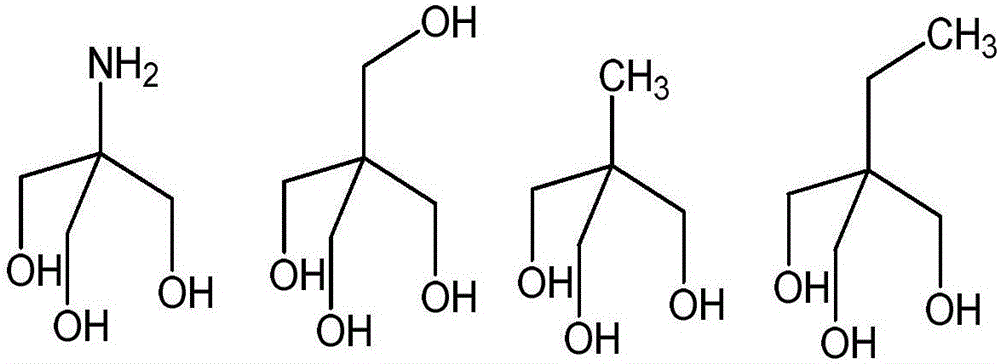
[0028] Example 1 (TRIS=trishydroxymethylaminomethane)
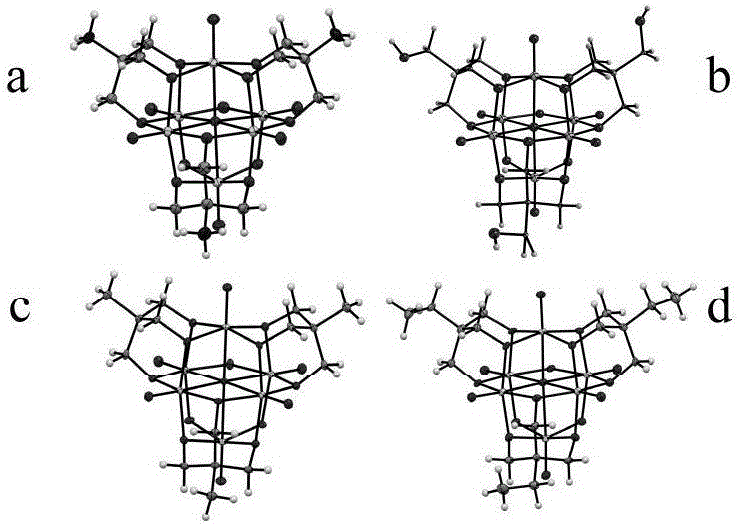
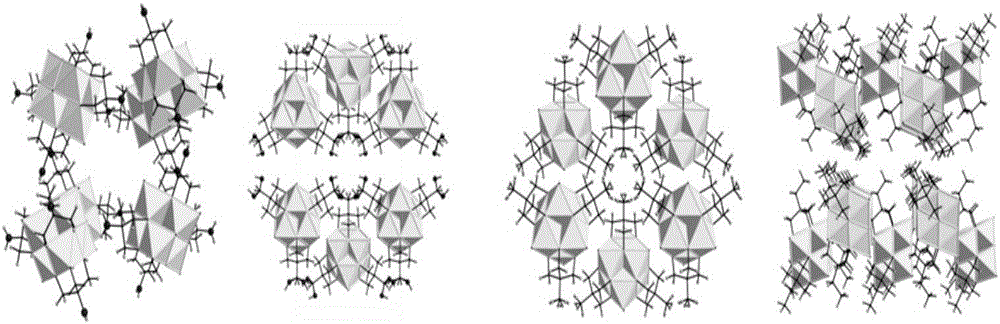
[0029] Compound 1 synthesis process: Weigh 1.6935g (14mmol) NaVO 3 , 0.8413g (7mmol) tris hydroxymethyl amino methane into the 50ml hydrothermal reaction kettle liner, then add 20ml deionized water and 150ul (4.6mmol) N 2 h 4 ∙ H 2 O, after stirring evenly, the reaction kettle was sealed and placed in an oven, reacted at 210°C for 24h, after cooling to room temperature, filtered the reaction mixture, and the filtrate was allowed to stand for 7 days to precipitate black blocky crystals. Use 20ml deionized water for crystals, 20ml anhydrous Naturally dry after washing with ethanol. IR (KBr-pellets, cm -1 ): 3475(s), 1608(m), 1534(m), 1111(s), 1049(s), 947(s), 745(s), 613(s); UV-Vis (H 2 O): λ max =347nm; Elemental analysis (%): theoretical value V 6 N 3 o 29 C 12 h 46 : N 4.19, C 14.38, H4.63; Test values: N 4.48, C 14.53, H 4.87
Embodiment 2
[0030] Example 2 (TRIS=pentaerythritol)
[0031] Compound 2 synthesis process: Weigh 1.6935g (14mmol) NaVO 3 , Add 0.9455g (7mmol) pentaerythritol into 50ml reactor liner, then add 20ml deionized water and 150ul (4.6mmol) N 2 h 4 ∙H 2 O, the reaction kettle was placed in an oven at 210°C for 24 hours. After cooling to room temperature, the reaction mixture was filtered, and the filtrate was allowed to stand for 7 days to precipitate blue flaky crystals. IR (KBr-pellets, cm -1 ): 3600(w), 3311(s), 1620(m), 1126(s), 1066(sh), 1033(s), 956(sh), 943(s), 798(w), 653(s ); UV-Vis (H 2 O): λ max =346nm; Elemental analysis (%): theoretical value forNa2V6O24C15H34: C 18.96, H 3.61; test value: C 19.52, H 3.21.
Embodiment 3
[0032] Example 3 (TRIS=trimethylolethane)
[0033] Compound 3 synthesis process: Weigh 1.6935g (14mmol) NaVO 3 , Add 0.8344g (7mmol) trimethylolethane into 50ml reactor liner, then add 20ml deionized water and 150ul (4.6mmol) N 2 h 4 ∙ H 2 O, the reaction kettle was placed in an oven at 210°C for 24 hours, and after cooling to room temperature, blue columnar crystals were precipitated. IR (KBr-pellets, cm -1 ):3594(w),2964(w),2893(m),2850(m),1403(m),1144(s),1047(s),974(s),943(sh),926(s ),847(s),617(s); UV-Vis (H 2 O): λmax=346nm; Elemental analysis (%): theoretical value V6N3O19C15H41: N 4.81, C20.63, H 4.73; test value: N 5.03, C 20.46, H 4.76.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com