Cirrhosis ascites treatment medicine
A cirrhotic ascites and drug technology, applied in the field of medicine, can solve the problems of water and electrolyte disorders, long-term use, slow curative effect of traditional Chinese medicine, etc., and achieve the effect of relieving pain, improving liver function, and quick results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
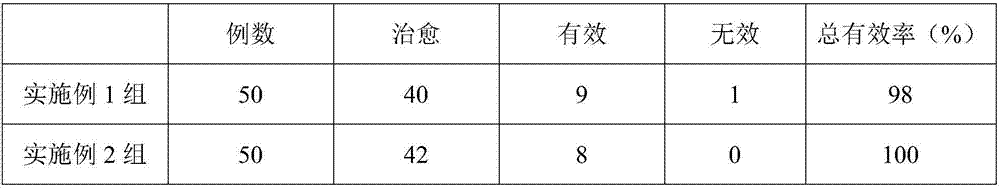
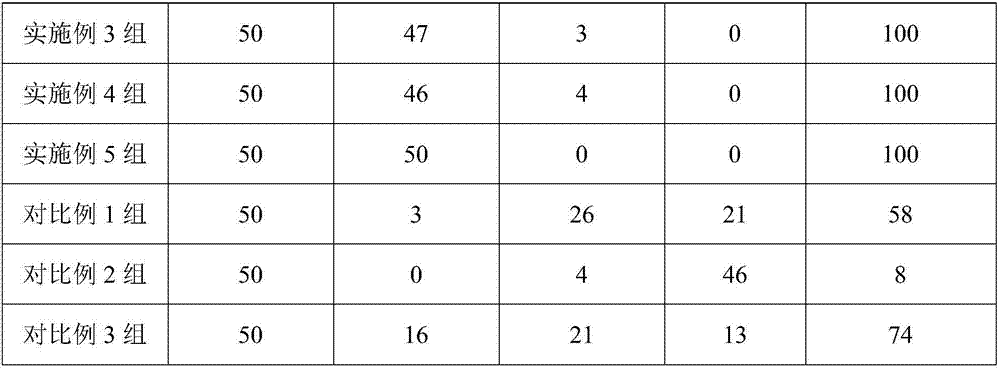
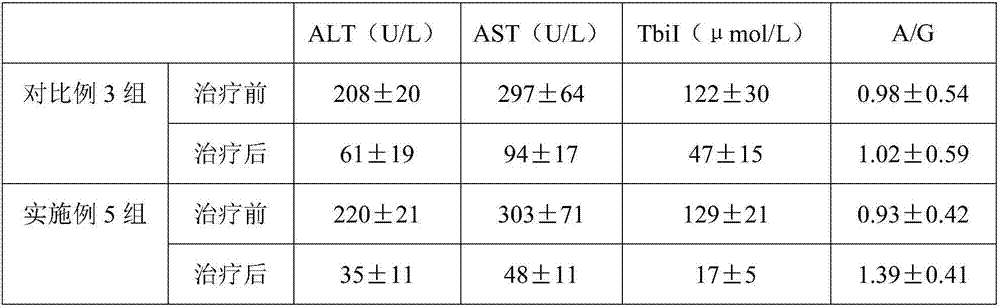
Examples
Embodiment 1
[0016] In an embodiment of the present invention, a medicine for treating liver cirrhosis ascites is composed of the following raw materials in parts by weight: 7 parts of rutin, 1 part of benzbromarone, 11 parts of glycerin, and 3 parts of naringin.
[0017] Mix and grind benzbromarone and glycerol, pass through a 120-mesh sieve, add deionized water 3.7 times the mass of the two, then raise the temperature to 81°C, and stir at this temperature for 36 minutes to prepare mixture A. Put naringin and rutin into the mixture A, then ultrasonically treat at 56°C for 28 minutes with an ultrasonic power of 1000W, then stir at 61°C until dry, and granulate to obtain the drug.
Embodiment 2
[0019] In an embodiment of the present invention, a medicine for treating liver cirrhosis ascites is composed of the following raw materials in parts by weight: 15 parts of rutin, 5 parts of benzbromarone, 19 parts of glycerin, and 7 parts of naringin.
[0020] Mix and grind benzbromarone and glycerol, pass through a 120-mesh sieve, add deionized water 3.7 times the mass of the two, then raise the temperature to 83°C, and stir at this temperature for 39 minutes to prepare mixture A. Put naringin and rutin into the mixture A, then ultrasonically treat at 56°C for 28 minutes with an ultrasonic power of 1000W, then stir at 61°C until dry, and granulate to obtain the drug.
Embodiment 3
[0022] In an embodiment of the present invention, a medicine for treating liver cirrhosis ascites is composed of the following raw materials in parts by weight: 9 parts of rutin, 2 parts of benzbromarone, 13 parts of glycerin, and 4 parts of naringin.
[0023] Mix and grind benzbromarone and glycerol, pass through a 120-mesh sieve, add deionized water 3.7 times the mass of the two, then raise the temperature to 82°C, and stir at this temperature for 37 minutes to prepare mixture A. Put naringin and rutin into the mixture A, then ultrasonically treat at 56°C for 28 minutes with an ultrasonic power of 1000W, then stir at 61°C until dry, and granulate to obtain the drug.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com