Circulating production process for preparing hydrogen fluoride by utilizing fluorosilicic acid
A production process, fluosilicic acid technology, applied in the direction of fluorine/hydrogen fluoride, hydrogen fluoride, silicon oxide, etc., can solve the problems of high yield, low value of by-products, low cost, etc., and achieve the effect of high yield and low process cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
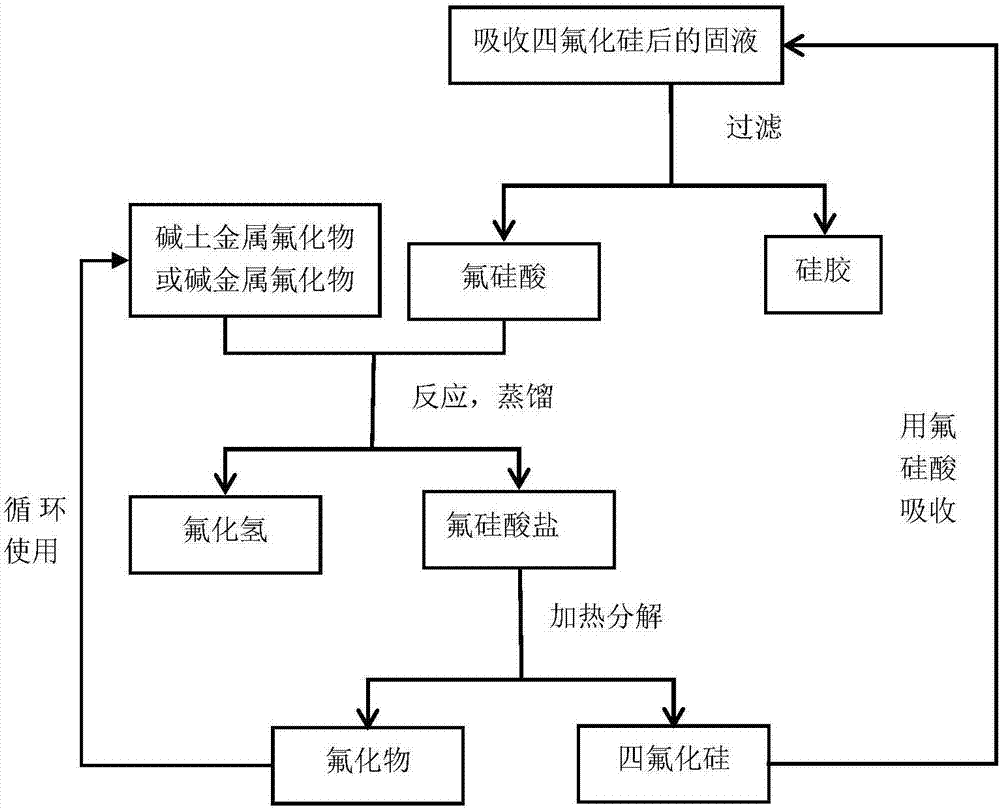
[0040] 1. The first production cycle
[0041] 1) 10 kg of fluorosilicate with a mass fraction of 20% and 0.73 kg of lithium fluoride solid are placed in a distillation still to react to obtain a mixed solution of hydrogen fluoride and lithium fluorosilicate;
[0042] 2) Vacuum distillation in the reaction kettle at 95 degrees Celsius for 1.5h, distilling hydrogen fluoride gas to obtain lithium fluorosilicate solid;
[0043] 3) Take out the solid in step 2) and heat to 450 degrees centigrade, heat for 1.5h to obtain lithium fluoride solid and silicon tetrafluoride gas; use 3.3kg mass fraction of 20% fluosilicic acid solution to absorb, so that the concentration of fluosilicic acid solution Elevate, and generate silica gel, filter the silica gel, and keep the fluorosilicic acid solution for later use;
[0044] 2. The second production cycle
[0045] 1) Fluosilicic acid obtained after absorbing silicon tetrafluoride in step 3) of the first production cycle and lithium fluoride ...
Embodiment 2
[0052] 1. The first production cycle
[0053] 1) Take 10 kg of fluorosilicate with a mass fraction of 15% and 1.27 kg of lithium fluoride solids and place them in a distillation pot for reaction to obtain a suspension mixture of hydrogen fluoride, lithium fluorosilicate and the remaining excess lithium fluoride;
[0054] 2) Distill at 180 degrees Celsius for 1 hour in a reaction kettle to distill hydrogen fluoride gas to obtain lithium fluorosilicate and lithium fluoride solids;
[0055] 3) Take out the solid in step 2) and heat it to 550 degrees centigrade, and heat for 1h to obtain lithium fluoride solid and silicon tetrafluoride gas; use 3.3kg of 15% fluosilicic acid solution to absorb it, so that the concentration of the fluosilicic acid solution rises High, and produce silica gel, fluosilicic acid solution is reserved for later use.
[0056] 2. The second production cycle
[0057] 1) Fluorosilicic acid obtained after absorbing silicon tetrafluoride in step 3) of the fir...
Embodiment 3
[0064] 1. The first production cycle
[0065] 1) Take 10 kg of fluorosilicate with a mass fraction of 10% and 1.08 kg of lithium fluoride solid and place them in a distillation still to react to obtain a suspension mixture of hydrogen fluoride, lithium fluorosilicate and the remaining excess lithium fluoride;
[0066] 2) Distill at 150 degrees Celsius for 2 hours in a reaction kettle to distill hydrogen fluoride gas to obtain a solid mixture of lithium fluorosilicate and lithium fluoride;
[0067] 3) Take out the solid in step 2) and heat to 300 degrees centigrade, heat for 3 hours to obtain lithium fluoride solid and silicon tetrafluoride gas; use 3.3 kg of 10% fluorosilicic acid solution to absorb, so that the concentration of fluorosilicic acid solution rises High, and produce silica gel, fluosilicic acid solution is reserved for later use.
[0068] 2. The second production cycle
[0069] 1) Fluorosilicic acid obtained after absorbing silicon tetrafluoride in step 3) of t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com