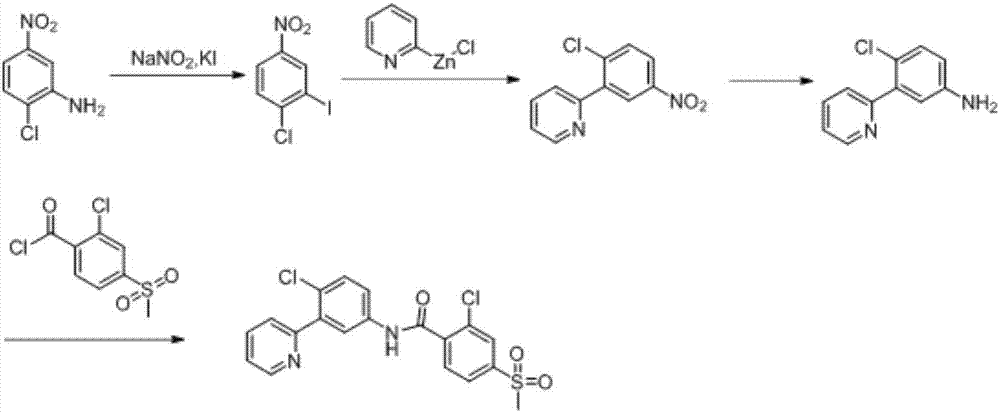
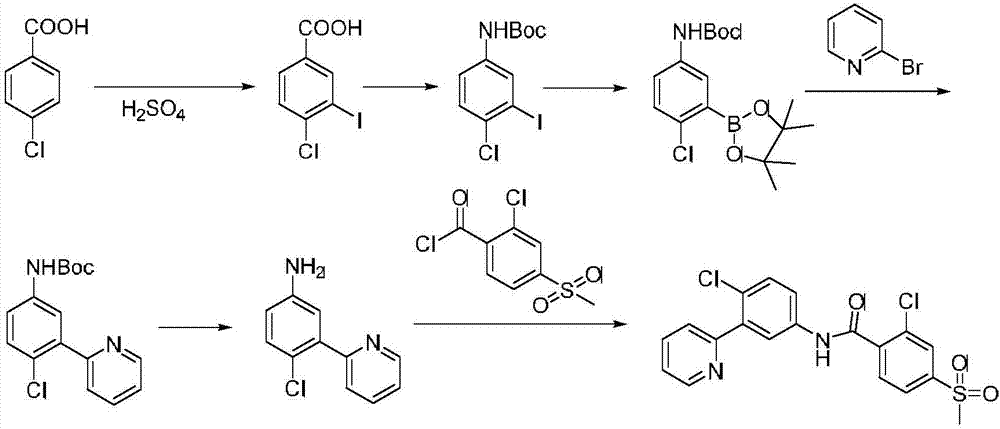
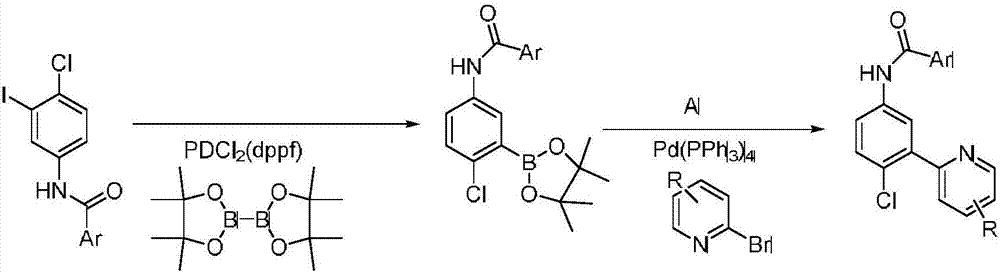
Synthesis method of vismodegib
A synthetic method, the technology of vemodinil, applied in the field of vemodinib synthesis, can solve the problems of difficult to obtain raw materials, harsh reaction conditions, long reaction steps, etc., and achieve small amount of reducing agent, short synthetic route, Environmentally friendly effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0028] The present invention will be described in detail below through examples.
[0029] 1. Synthesis of Intermediate 2
[0030]
[0031] In the reaction tube, add 2-chloro-5-nitrophenylboronic acid (0.201g, 1mmol), potassium carbonate (0.138g, 1mmol, 1eq), palladium acetate (0.0112g, 0.05mmol, 5mol%), isopropanol ( 5mL) and water (5mL) in a mixed solvent, after the solid was dissolved, 2-bromopyridine (0.174g, 1.1mmol, 1.1eq) was added dropwise under stirring, reacted at 25°C for 3h, and extracted with ethyl acetate Three times, the obtained organic phases were combined together, added silica gel to spin dry, dry-packed the column, and passed the column with petroleum ether: ethyl acetate (v:v=1:1) to obtain the product intermediate 2-(2-chloro-5 -Nitrophenyl)pyridine (2) 0.219g, yield 96%.
[0032] 2. Synthesis of Intermediate 3
[0033]
[0034] Add intermediate 2-(2-chloro-5-nitrophenyl)pyridine (2) (0.114g, 0.5mmol), reducing agent Pd / C (5%Pd) (0.020g), organic ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com