Neonatal microbiome supplementation
A microbiological, neonatal technology, applied in the field of improving the chances of life outside the womb, supplementing the microbiome of newborns, can solve the problem of NEC without effective cure or treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0095] Manufacture of chewing gum products containing probiotic strains
[0096] In this example, Lactobacillus reuteri DSM 17938 or ATCC 5289 (FJ1) was chosen for addition of the strains to chewing gum. Lactobacillus reuteri was grown and lyophilized using standard methods used in the industry for growing Lactobacilli.
[0097] The steps of an example manufacturing method of chewing gum comprising selected strains are as follows, it being understood that excipients, fillers, flavours, encapsulators, lubricants, anti-caking agents, sweeteners and other components of the chewing gum product as known in the art Components can be used without affecting product efficacy:
[0098] 1 melted. Softisan 154 (SASOL GMBH, Bad Homburg, Germany) was melted in a vessel and heated to 70°C to ensure complete fragmentation of the crystal structure. It is then cooled to 52 - 55°C (just above its hardening point).
[0099] 2 Granulation. Transfer the L. reuteri freeze-dried powder to a Di...
Embodiment 2
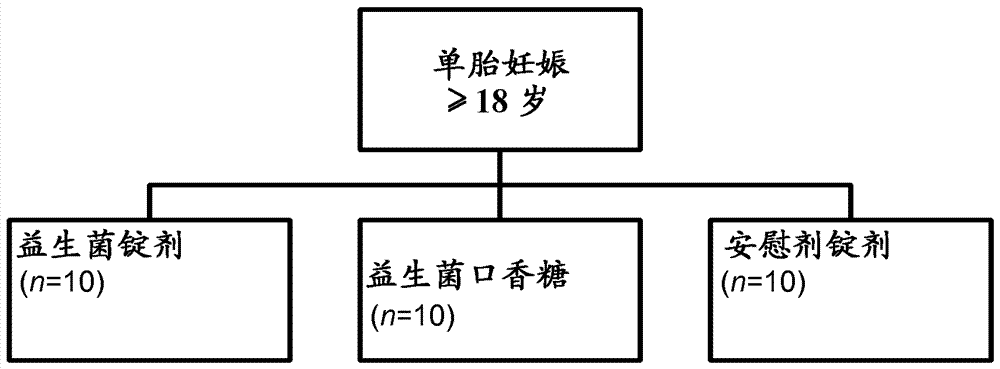
[0108] A test group of pregnant women was included in one study. A subgroup of the test group received L. reuteri strain ATCC PTA 5289 in the form of lyophilized powder in standard gelatin capsules, while the other subgroup of the test group received L. reuteri DSM 17938 in the form of chewing gum. The probiotics were given during the women's last trimester of pregnancy. Lactobacillus reuteri strain ATCC PTA 5289 is in gelatin capsule form because the desired probiotic effect occurs in the mother's digestive tract. On the other hand, Lactobacillus reuteri DSM 17938 was orally administered to the mother in the form of chewing gum to maximize the exposure time and site-specific exposure in the oral cavity, and thus successfully colonized the oral cavity. After colonization in the mother's mouth, the probiotic bacteria can then disseminate via the bloodstream to the placenta and further to the baby's digestive tract.
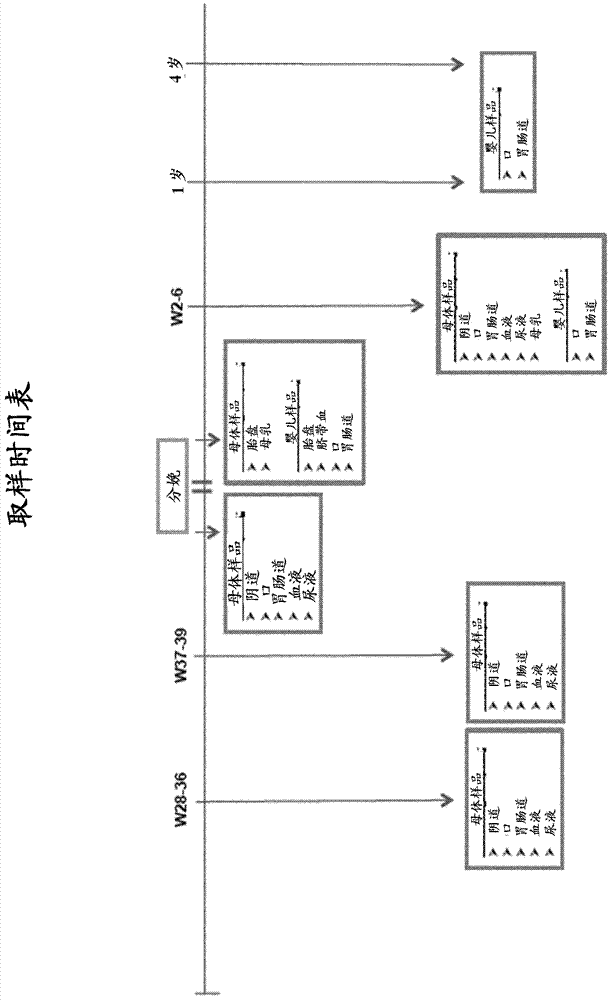
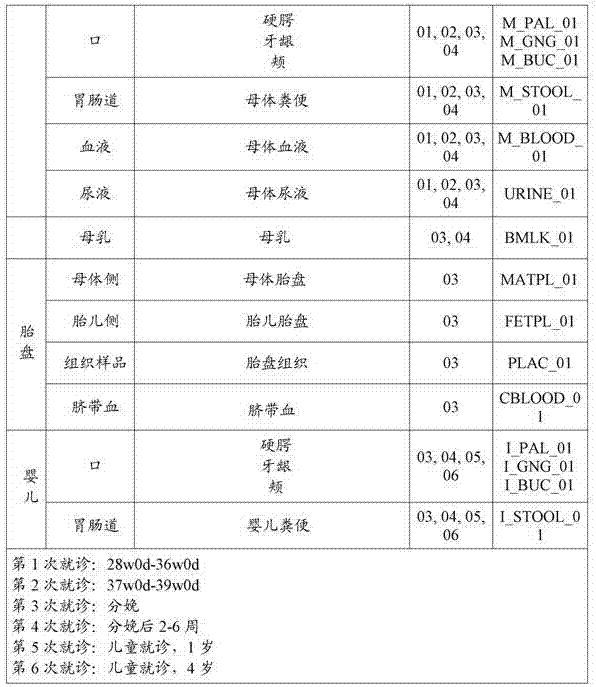
[0109] Fecal samples may be collected from each subject on ...
Embodiment 3
[0114] Pregnant mothers treated with probiotics to reduce risk of bearing children with suboptimal gastrointestinal microbiomes. Pregnant mothers will take oral probiotics during their pregnancy, preferably certain lactic acid bacteria such as Lactobacillus reuteri DSM 17938, and thereby transfer them to the fetus through the blood and placenta.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com