Aluminum-air battery electrolyte and preparation thereof
An air battery and electrolyte technology, applied in primary batteries, alkaline electrolytes, aqueous electrolytes, etc., can solve the problems of reduced Coulombic efficiency, anode corrosion and hydrogen evolution, and restrictions on the commercial development of alkaline aluminum-air batteries. Battery life, increase discharge power, and reduce the effect of anode hydrogen evolution self-corrosion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Weigh and mix 0.70g of sodium stannate, 0.08g of casein, 0.01g of polyoxyethylene sorbitan monostearate and 0.03g of sorbitan monostearate in the corrosion inhibitor, add to 50mL 4mol / LNaOH solution, stirred in a constant temperature water bath at 70°C for 10 minutes to dissolve evenly, and cooled to room temperature to obtain an alkaline electrolyte containing an anode corrosion inhibitor for alkaline aluminum-air batteries.
[0022] The static hydrogen evolution self-corrosion rate of the Al-0.2Mg-0.11Ga-0.1Sn alloy in the electrolyte prepared in this embodiment was tested by the gas collection experiment. The test time was 60 minutes. The results are shown in Table 1.
Embodiment 2
[0024] Mix 0.64g of sodium stannate, 0.08g of casein, and 0.04g of polyoxyethylene sorbitan monostearate in the corrosion inhibitor, add to 50mL of 4mol / L NaOH solution, stir in a constant temperature water bath at 70°C for 10 minutes, Dissolve evenly and cool to room temperature to obtain an electrolyte containing an anode corrosion inhibitor for alkaline aluminum-air batteries.
[0025] The static hydrogen evolution self-corrosion rate of the Al-0.2Mg-0.11Ga-0.1Sn alloy in the electrolyte prepared in this embodiment was tested by the gas collection experiment. The test time was 60 minutes. The results are shown in Table 1.
Embodiment 3
[0027] Mix 0.54g of sodium stannate, 0.06g of casein, 0.01g of polyoxyethylene sorbitan monostearate and 0.01g of sorbitan monostearate in the corrosion inhibitor, and add to 50mL 4mol / L NaOH solution , Stir in a constant temperature water bath at 70°C for 10 minutes to dissolve evenly, and cool to room temperature to obtain an electrolyte containing an anode corrosion inhibitor for an alkaline aluminum-air battery.
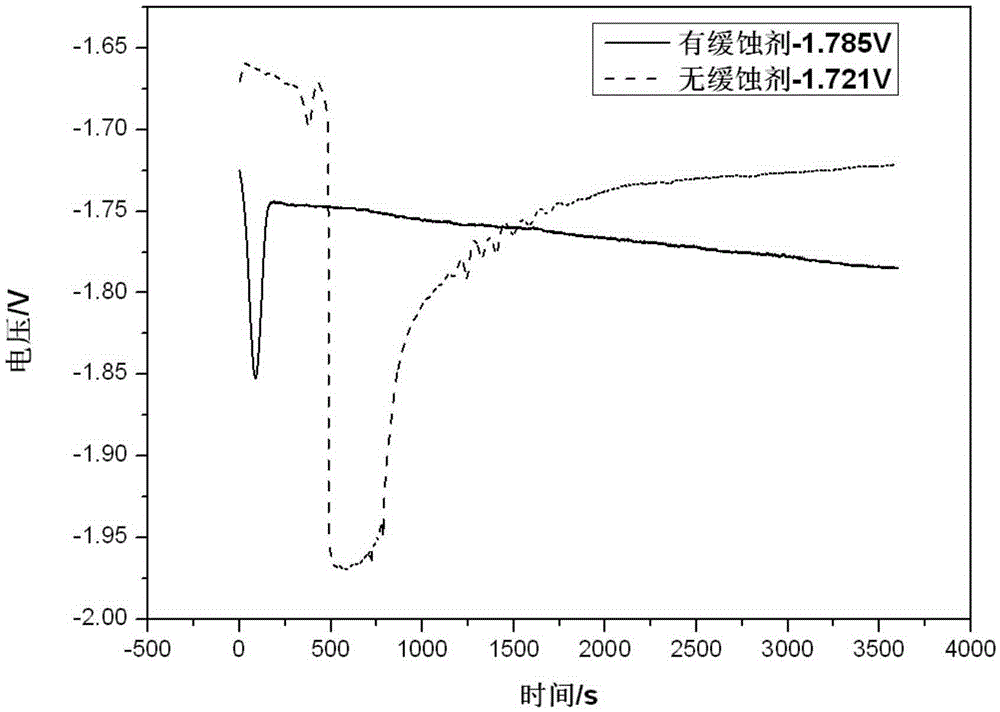
[0028] The static hydrogen evolution self-corrosion rate of the Al-0.2Mg-0.11Ga-0.1Sn alloy in the electrolyte prepared in this embodiment was tested by the gas collection experiment. The test time was 60 minutes. The results are shown in Table 1. Using a three-electrode system to measure the open circuit potential of the alloy in the above electrolyte at room temperature, the results are detailed in figure 1 .
[0029] from figure 1 It can be seen that the open circuit potential of the above-mentioned alloy in the electrolyte of this embodiment is -1.785V, whi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com