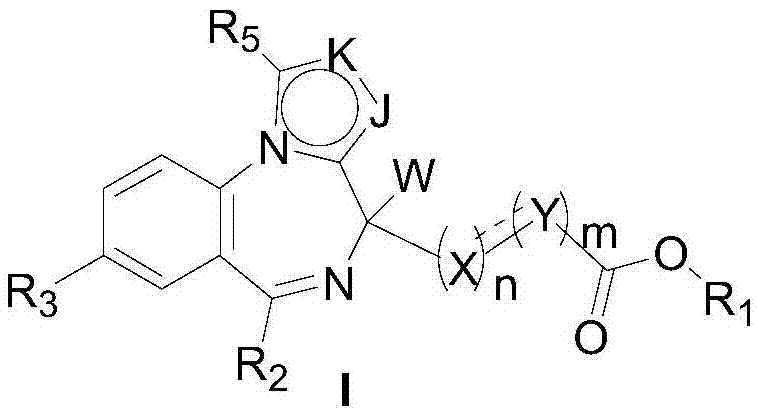
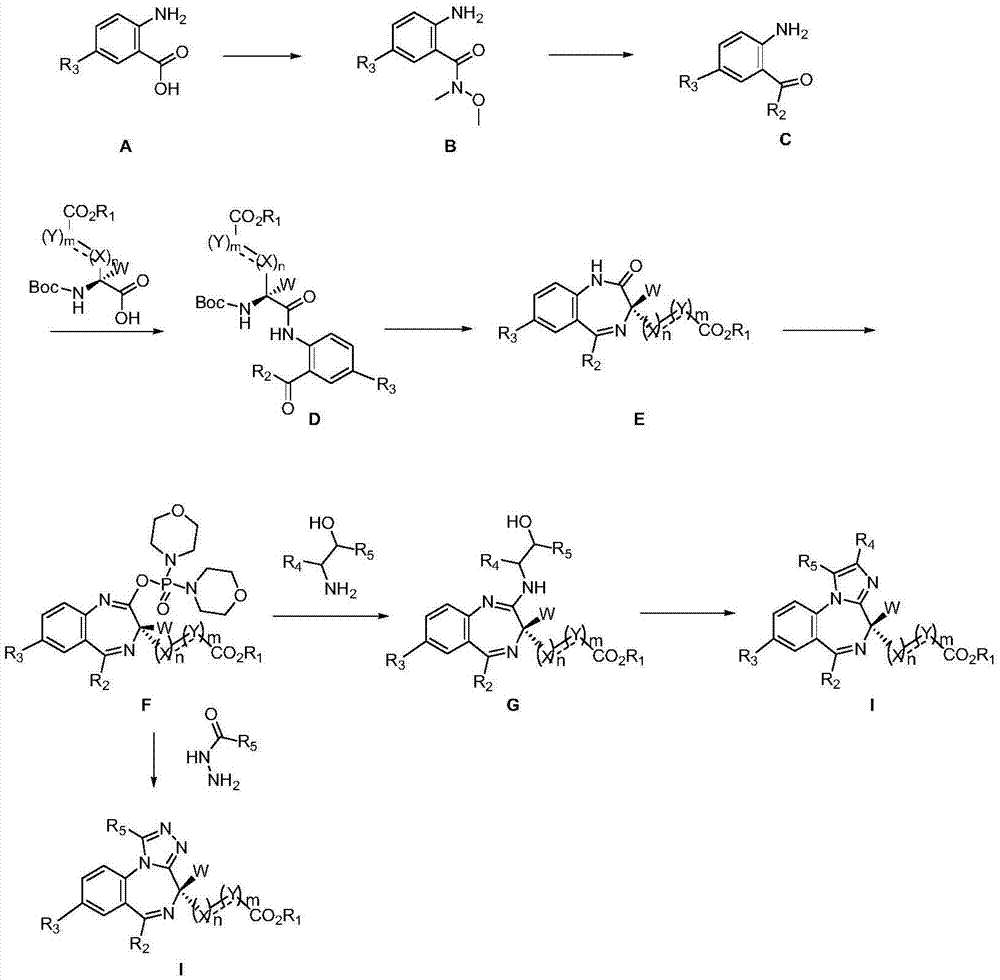
Short-acting benzodiazepine derivatives, and preparation methods and uses thereof
A technology for compounds and polymorphs, applied in the field of benzodiazepine derivatives, can solve the problems of long time to regain sobriety, drug-drug interactions, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0257] Example 1: 3-(8-bromo-1-methyl-6-(pyridin-2-yl)-4H-benzo[f]imidazo[1,2-a][1,4]diazepine -4-yl) methyl acrylate (compound 1)
[0258]
[0259] The first step: the preparation of 2-bromo-N-[4-bromo-2-(pyridin-2-ylcarbonyl)phenyl]acetamide (compound 1b)
[0260] The compound 2-(2-amino-5-bromo-benzoyl)pyridine (Compound 1a, 60 g, 0.22 mol) was dissolved in DCM (3 L) and NaHCO was added 3 (36.9 g, 0.44 mol). The mixture was cooled to 0°C, and bromoacetyl bromide (52.4 g, 0.26 mol) was slowly added dropwise. Stir at 0°C for 2 hours until TLC shows the reaction is complete. After the reaction solution was concentrated, the target product 2-bromo-N-[4-bromo-2-(pyridin-2-ylcarbonyl)phenyl]acetamide (compound 1b, 88 g, yield: 100.0%) was obtained.
[0261] The second step: 7-bromo-5-(pyridin-2-yl)-1H-benzo[e][1,4]diazepine Preparation of -2(3H)-one (compound 1c)
[0262] Compound 2-bromo-N-[4-bromo-2-(pyridin-2-ylcarbonyl)phenyl]acetamide (Compound 1b, 88 g, 0.22 mol)...
Embodiment 2
[0275] Example 2: (S)-3-(8-bromo-6-(pyridin-2-yl)-4H-benzo[f]imidazo[1,2-a][1,4]diazepine -4-yl) methyl propionate (compound 2s)
[0276]
[0277] The first step: (S)-5-((4-bromo-2-nicotinoylphenyl)amino)-4-((tert-butoxycarbonyl)amino)-5-oxopentanoic acid methyl ester (compound 2b) preparation of
[0278] Add HATU (45.6g, 0.12mol), N-methylmorpholine (20.2g, 0.2mol) and N-Boc-L-glutamic acid-5-methyl ester (16.1g , 0.1 mol), and the resulting mixture was allowed to react in an ice bath for 30 minutes, and then (2-amino-5-bromophenyl)(pyridin-2-yl)methanone (compound 2a, 27.7g, 0.1 mol) was added. After the TLC monitoring reaction was complete, water was added to the reaction system, extracted with ethyl acetate (20mL x 4), the ethyl acetate layer was evaporated to dryness, and the residue was purified by column chromatography to obtain the target product (S)-5-((4 -Bromo-2-nicotinoylphenyl)amino)-4-((tert-butoxycarbonyl)amino)-5-oxopentanoic acid methyl ester (compound ...
Embodiment 3
[0288] Example 3: (S)-3-(8-chloro-6-phenyl-4H-benzo[f][1,2,4]triazolo[4,3-a][1,4] Diazepines -4-yl) methyl propionate (compound 3s)
[0289]
[0290] The first step: (S)-5-((2-benzoyl-4-chlorophenyl)amino)-4-((tert-butoxycarbonyl)amino)-5-oxopentanoic acid methyl ester (compound 3c ) preparation
[0291] (2-Amino-5-chlorophenyl)(benzyl)methanone (compound 3a, 5g, 0.022mol) and compound N-tert-butoxycarbonyl-L-glutamic acid-5-methyl ester (compound 3b, 6.32 g, 0.024 mol) was dissolved in DCM (50 mL). The mixture was cooled to 0 °C, DCC (4.99 g, 0.024 mmol) was added, and stirred for 24 hours. LCMS showed the reaction was complete. The reaction solution was poured into ice water, extracted with ethyl acetate, the organic phase was washed with water, dried and concentrated, and the residue was purified by silica gel column chromatography to obtain the target product (S)-5-((2-benzoyl -methyl 4-chlorophenyl)amino)-4-((tert-butoxycarbonyl)amino)-5-oxopentanoate (compound ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com