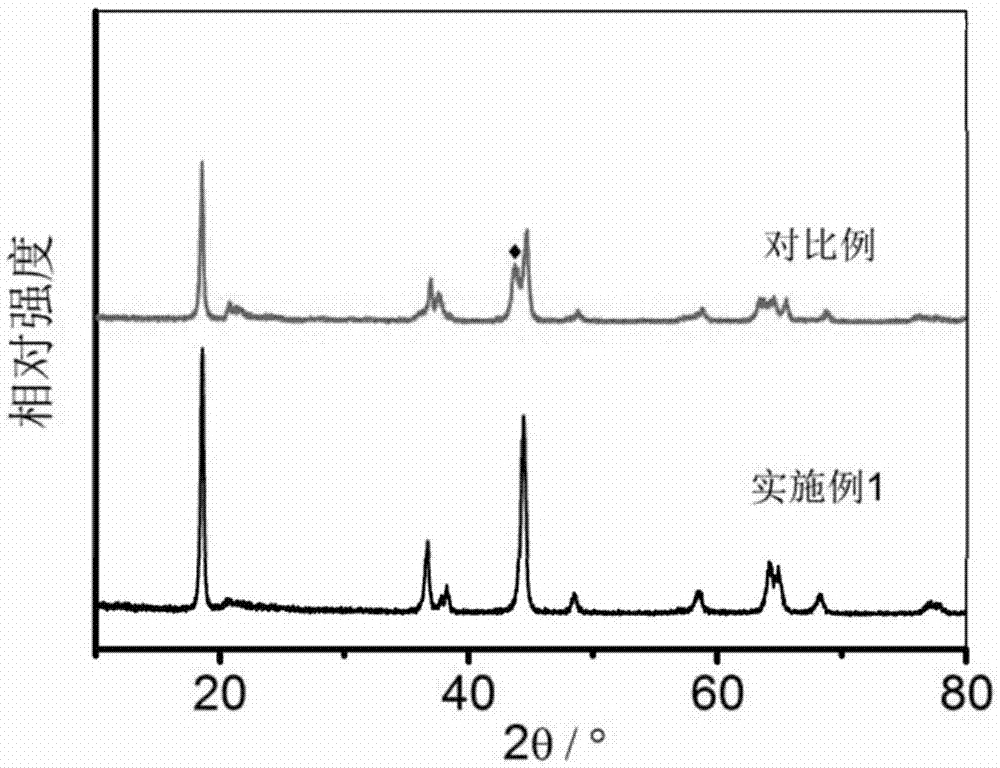
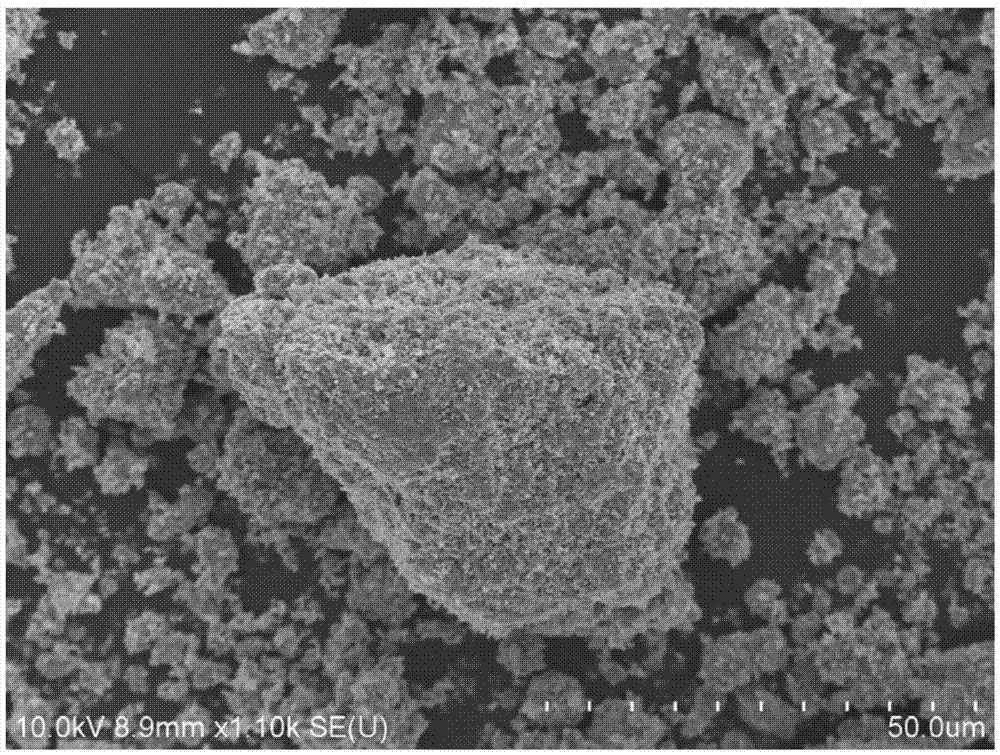
Lithium-rich manganese-based anode material, preparation method thereof and lithium ion battery containing anode material
A lithium-rich manganese-based, cathode material technology, applied in battery electrodes, secondary batteries, circuits, etc., can solve the problems of uncontrollable material morphology, particle size and precise chemical composition, to improve efficiency and operability, The effect of simplifying the process flow and saving washing water
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] (1) According to the molecular formula Li 1.2 mn 0.54 Ni 0.13 co 0.11 Al 0.02 o 2 The molar ratio of the transition metal in aluminum acetate, nickel acetate, cobalt acetate and manganese acetate is configured so that the total transition metal ion concentration is 1.0mol L -1 The mixed solution of Al: Co: Ni: Mn = 0.025: 0.140: 0.165: 0.67; the configuration is 1.0mol L -1 oxalic acid solution, configure 1mol L -1 NH 4OH solution, the three solutions are dripped into the constantly stirring reactor at the same time at a certain flow rate; the reaction conditions are controlled: the temperature is 60°C, the stirring speed is 1200rpm, and the co-precipitation reaction time is 10h;
[0034] (2) Adjust the solid concentration of the resulting slurry to 150g / L, and spray dry at 160°C to obtain the precursor Ni 0.165 co 0.140 Al 0.025 mn 0.67 C 2 o 4 2H 2 O;
[0035] (3) keeping the above-mentioned oxalate precursor powder at 320°C for 4 hours, and then raisin...
Embodiment 2
[0041] (1) According to the molecular formula Li 1.16 mn 0.56 Ni 0.16 co 0.08 f 0.02 o 1.98 The molar ratio of the transition metal in nickel acetate, cobalt acetate and manganese acetate is configured so that the total transition metal ion concentration is 1.0mol L -1 The mixed solution, wherein, Ni: Co: Mn = 0.2: 0.1: 0.7; configured as 1.0mol L -1 NH 4 HC 2 h 4 Solution, configure 0.5mol·L -1 NH 4 OH solution; drop the above three solutions into a constantly stirring reactor at a certain flow rate at the same time, and control the reaction conditions: the reaction temperature is 80°C, the stirring speed is 800rpm, and the reaction time is 10h;
[0042] (2) Adjust the solid concentration of the resulting slurry to 250g / L, and spray dry at 170°C to obtain the precursor Ni 0.2 co 0.1 mn 0.7 C 2 h 4 2H 2 O;
[0043] (3) heat the above precursor powder at 200°C for 4 hours, then raise the temperature to 400°C for 3 hours to obtain the oxide precursor;
[0044] ...
Embodiment 3
[0048] (1) According to the molecular formula Li 1.167 mn 0.533 Ni 0.2 co 0.1 o 2 The molar ratio of the transition metal in nickel acetate, cobalt acetate and manganese acetate is configured so that the total transition metal ion concentration is 1.0mol L -1 The mixed solution of Ni:Co:Mn=0.24:0.12:0.64, the configuration is 1.0mol L -1 of (NH 4 ) 2 C 2 h 4 Solution, configure 4mol·L -1 NH 4 OH solution; drop the above three solutions into a constantly stirring reactor at a certain flow rate at the same time, and control the reaction conditions: the reaction temperature is 30°C, the stirring speed is 400rpm, and the reaction time is 4h;
[0049] (2) Adjust the solid concentration of the obtained slurry to 300g / L, and spray dry at 180°C to obtain the precursor Ni 0.24 co 0.12 mn 0.64 C 2 h 4 .2H 2 O;
[0050] (3) keeping the above precursor powder at 150°C for 4 hours, then raising the temperature to 450°C for 3 hours to obtain the oxide precursor;
[0051] (...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com