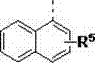
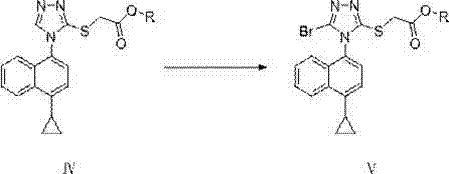
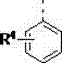
Quaternary ammonium salt catalysis and applications
A catalyst and reaction technology, applied in the field of medicine, can solve the problems of difficult post-processing, low yield, high cost and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] The preparation of embodiment 1 catalyst b1
[0026]
[0027] Dissolve compound a1 (50g, 147.3mmol, 1.0eq.) in 500mL of acetone, stir to dissolve at 20~30°C, add methyl bromoacetate (1473mmol, 10eq.), react for 12 hours, and concentrate the reaction solution to dryness , the concentrate was recrystallized from ethyl acetate to obtain 56.4 g of catalyst b1, yield: 77.8%.
[0028] 1 H NMR (CDCl 3 , 500 MHz) δ 11.65 (s, 1H), 8.59 (d, 1H, J = 8.0 Hz),7.66-7.73 (m, 3H), 7.34-7.44 (m, 2H), 6.15(d, 1H, J = 16.5 Hz), 5.69 (d, 1H, J = 16.5 Hz), 3.98-4.10 (m, 2H), 3.87 (s,3H), 3.77 (s,3H), 2.40-2.49 (m, 1H),1.25-1.31 (t, 3H, J = 7.0 Hz), 1.14-1.24 (m, 2H), 0.78-0.95(m, 2H).
[0029] 13 C NMR (CDCl 3 , 125 MHz) δ 166.1, 165.1, 155.9, 147.0, 145.6, 134.3,128.8, 127.8, 127.5, 125.7, 125.6, 124.0, 123.0, 120.9, 53.7, 52.3, 52.6, 7.1,
[0030] ESI-MS [M] + m / z 426.
Embodiment 2
[0031] The preparation of embodiment 2 catalyst b2
[0032]
[0033] Dissolve compound a2 (50g, 141.5mmol, 1.0eq.) in 500mL of acetone, stir to dissolve at 20~30°C, add ethyl bromoacetate (1415mmol, 10eq.), react for 12 hours, and concentrate the reaction solution to dryness , the concentrate was recrystallized from ethyl acetate to obtain 53.1 g of catalyst b2, yield: 72.2%.
[0034] 1 H NMR (CDCl 3 , 500 MHz) δ 11.63 (s, 1H), 8.57 (d, 1H, J = 8.0 Hz),7.65-7.72 (m, 3H), 7.34-7.41 (m, 2H), 6.15 (d, 1H, J = 16.5 Hz), 5.71 (d, 1H, J = 16.5 Hz), 4.30-4.37 (m, 2H), 4.18-4.25 (m, 2H), 3.98-4.10 (m, 2H), 2.40-2.49 (m, 1H), 1.32-1.39 (t, 3H, J = 7.0 Hz), 1.24-1.30 (t, 3H, J = 7.0 Hz),1.13-1.23 (m, 2H),0.77-0.94(m, 2H).
[0035] 13 C NMR (CDCl 3, 125 MHz) Δ 166.1, 165.1, 155.9, 147.0, 145.6, 134.3,128.8, 127.5, 125.7, 124.0, 123.0, 120.9, 62.8, 53.9.9, 13.5, 7.9, 6.9.
[0036] ESI-MS [M] + m / z 440.
Embodiment 3
[0037] The preparation of embodiment 3 catalyst b5
[0038]
[0039] Dissolve compound a5 (50g, 127.5mmol, 1.0eq.) in 500mL ethyl acetate, stir to dissolve at 25~35°C, add ethyl bromoacetate (1275mmol, 10eq.), react for 12 hours, and concentrate the reaction solution To dryness, the concentrate was recrystallized with ethyl acetate to obtain 57.4 g of catalyst b5, yield: 80.5%.
[0040] 1 H NMR (CDCl 3 , 500 MHz) δ 11.64 (s, 1H), 8.58 (d, 1H, J = 8.0 Hz),7.65-7.72 (m, 3H), 7.34-7.41 (m, 2H), 6.14 (d, 1H, J = 16.5 Hz), 5.70 (d, 1H, J = 16.5 Hz), 4.31-4.37 (m, 2H), 4.19-4.25 (m, 2H), 3.98-4.10 (m, 2H), 1.31-1.38 (t, 3H, J = 7.0 Hz), 1.23-1.29 (t, 3H, J = 7.0 Hz).
[0041] 13 C NMR (CDCl 3 , 125 MHz) δ 166.1, 165.1, 155.9, 147.0, 134.3, 128.8,127.8, 127.5, 125.7, 125.6, 124.0, 123.0, 120.9, 120.0, 63.3, 62.9, 53.9,13.5,
[0042] ESI-MS [M] + m / z 478.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com