Method for precipitating sponge copper powder through zinc-ammonia-ammonium salt solution system displacement
An ammonium salt solution and sponge technology, applied in the field of copper sponge deposition, can solve the problems of low current efficiency, complex equipment, and difficult operation of electrodeposited copper, and achieve high copper removal efficiency, reduced zinc consumption, and less impurities.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] 1. Using recycled brass smelting slag from a factory as raw material, 5mol / L ammonium chloride and 2mol / L ammonia solution are used as leaching agents, and the chemical composition (g / L) of the leaching solution is: Zn 2+ 57.87, Cu 2+ +Cu + 14.71, Ni 2+ 0.04, Co 2+ 0.05, Pb 2+ 0.01. Get this material liquid 2000ml and be placed in the reaction tank of 2500ml, length 20cm, width 10cm, the zinc plate of thickness 1mm is anode, length 24cm, width 14cm, the titanium plate of thickness 2mm is cathode.
[0035] 2. Put the reaction tank into a 35°C water bath, connect the zinc plate to the positive pole of the DC power supply, and connect the titanium plate to the negative pole of the DC power supply.
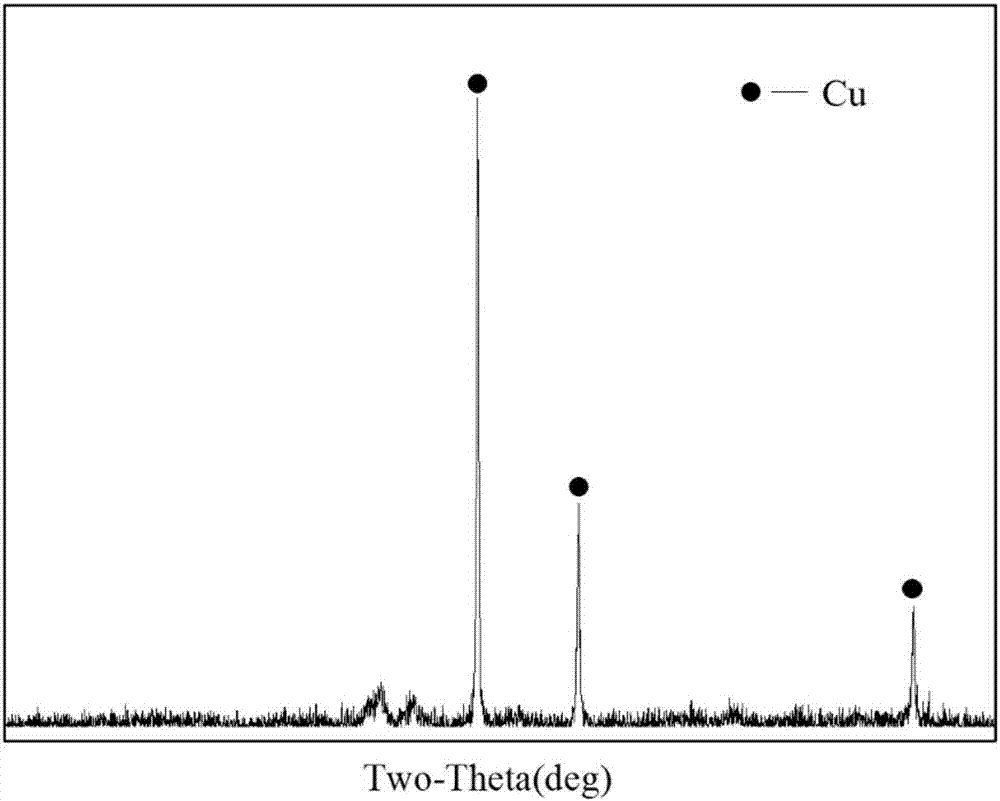
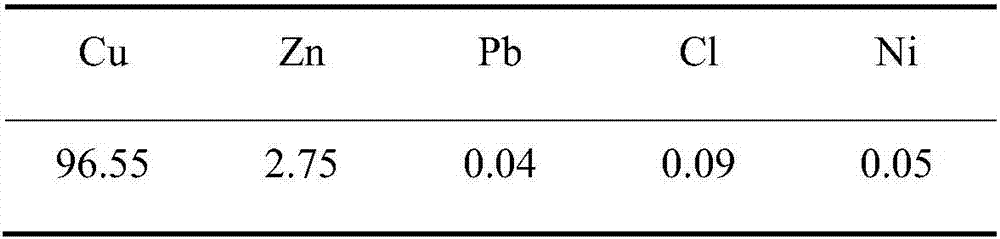
[0036] 3. Adjust the current density on the zinc plate to 200A / m 2 , the distance between the zinc plate and the titanium plate is 10cm, the potential difference between the zinc plate and the titanium plate is 0.46V, and the solution contains Cu 2+ 0.18g / L, the grade of...
Embodiment 2
[0044] 1. Using a factory's recycled brass smelting slag as raw material, using 5mol / L ammonium chloride solution as leaching agent, the chemical composition (g / L) of the leaching solution is: Zn 2+ 114.5, Cu 2+ +Cu + 6.76, Ni 2+ 0.06, Co 2+ 0.045, Sb 3+ 0.01, Cd 2+ 0.01, Pb 2+ 0.02. Take 2000ml of this feed solution and place it in a 2500ml reaction tank. A zinc plate with a length of 20cm, a width of 10cm, and a thickness of 1mm is used as the anode, and a titanium mesh with a length of 22cm, a width of 12cm, and a thickness of 2mm is used as the cathode. The anode and the cathode are polished smooth with sandpaper.
[0045] 2. Put the reaction tank into a 70°C water bath, connect the zinc plate to the positive pole of the DC power supply, and connect the titanium mesh to the negative pole of the DC power supply.
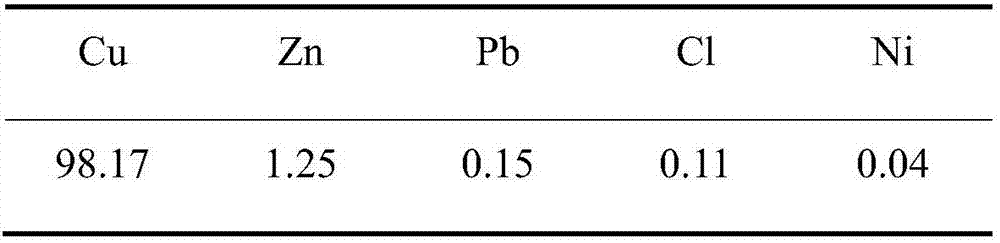
[0046] 3. Adjust the current density on the zinc plate to 70A / m 2 , the distance between the zinc plate and the titanium mesh is 5cm, the potential differ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com