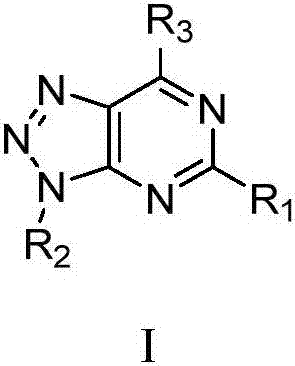
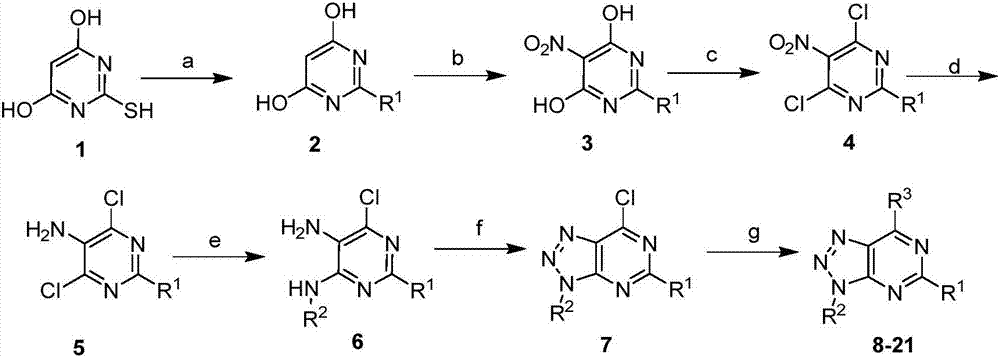
Pyrimidotriazole-containing LSD1 inhibitor, preparation method and application
A technology of triazole and inhibitor, applied in the field of medicinal chemistry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Compound 8 of Example 1, R 1 =propyl-S-, preparation of
[0052] (1) Compound 2 (R 1 = the preparation of propyl-S-)
[0053] Barbituric acid (3g, 1eq) and triethylamine (2.9ml, 1eq) were added to 30ml of methanol, under reflux, bromopropane (1.8ml, 1eq) was slowly added dropwise, and reflux was continued for 1 hour after the addition , cooled, and suction filtered to obtain 3.7g of pink solid compound 2b with a yield of 97%.
[0054] (2) Compound 3 (R 1 = the preparation of propyl-S-)
[0055] Under ice bath, carefully dissolve 3ml of fuming nitric acid in 6ml of acetic acid, then add 2.9g of compound 2b in batches, after the addition, continue to stir for 2 hours, then add the reaction solution to 18ml of ice water, filter with suction, After washing with water, a dark red powder compound 3b was obtained with a yield of 77.5%.
[0056] (3) Compound 4 (R 1 = the preparation of propyl-S-)
[0057] Compound 3b (12.4g, 1eq) was dissolved in 50ml of phosphorus ox...
Embodiment 2
[0066] Example 2 Compound 9, R 1 =propyl-S-, preparation of
[0067] The hydroxyethylamine of methanesulfonyl chloride protection replaces ethanolamine, adopts the same method of embodiment 7 to prepare 9. 1 HNMR (400MHz, CDCl 3 ,ppm): δ8.12-8.14(d,J=8.0Hz,1H),7.97-7.99(d,J=7.6Hz,1H),7.55-7.59(m,1H),7.49-7.53(m,1H ), 4.95-4.97(t, J=5.2Hz, 2H), 4.79-4.81(t, J=5.2Hz, 2H), 3.00(s, 3H), 1.57-1.62(m, 2H), 0.85-0.89( t,J=7.4Hz,3H). 13 CNMR (100MHz, CDCl 3 , ppm): δ171.34, 160.36, 154.99, 152.17, 149.24, 137.33, 131.06, 126.62, 126.08, 123.47, 121.31, 65.21, 46.06, 37.95, 33.50, 22.17, 13.26. Yield 70%.
Embodiment 3
[0068] Example 3 Compound 10, R 1 =propyl-S-, preparation of
[0069] The hydroxyethylamine of benzoyl protection replaces ethanolamine, adopts the same method of embodiment 7 to prepare 10. 1 HNMR (400MHz, CDCl 3 ,ppm): δ8.11-8.13(d,J=8.0Hz,1H),7.96-7.98(d,J=7.6Hz,1H),7.91-7.94(m,2H),7.54-7.58(m,2H ),7.48-7.52(m,1H),7.40-7.44(m,2H),5.02-5.05(t,J=5.2Hz,2H),4.83-4.85(t,J=5.2Hz,2H),2.87- 2.90(t,J=7.2Hz,2H),1.50-1.59(m,2H),0.83-0.86(t,J=7.4Hz,3H). 13 CNMR (100MHz, CDCl 3 ,ppm):δ171.04,166.04,160.02,155.33,152.08,149.33,137.23,133.37,131.01,129.75,129.20,128.50,126.57,125.99,123.40,121.26,62.34,46.12,33.32,22.12,13.27.。 Yield 63%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com