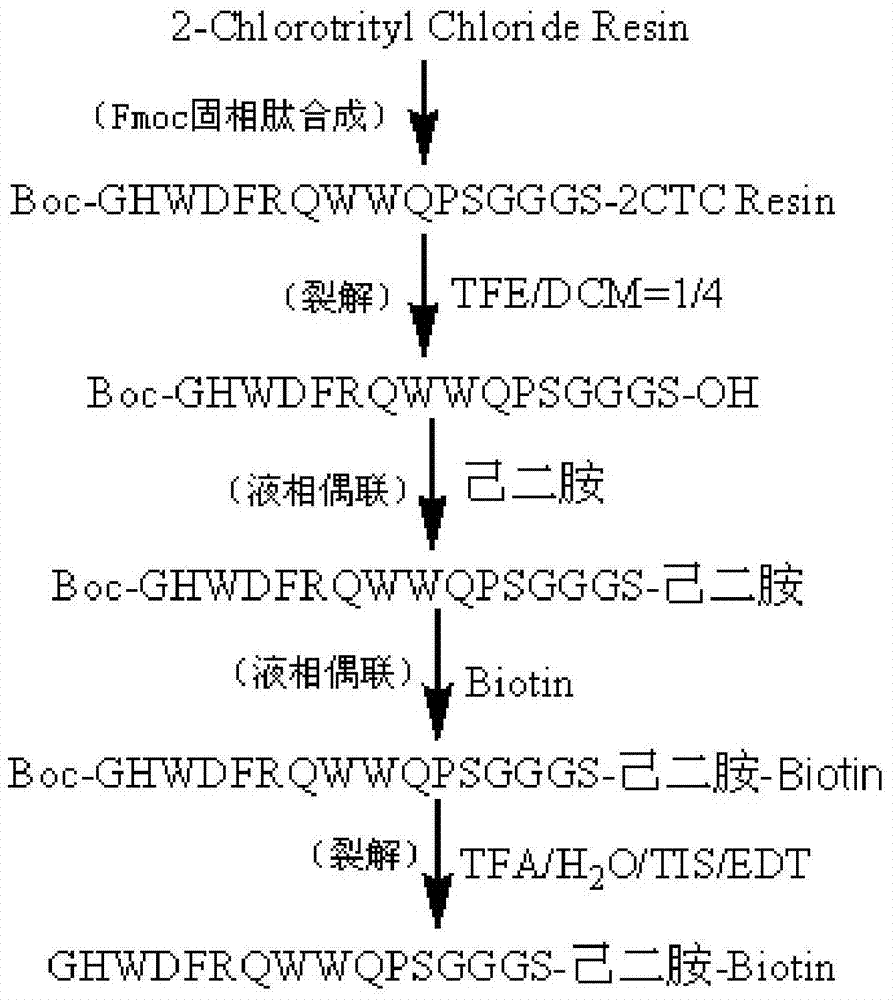
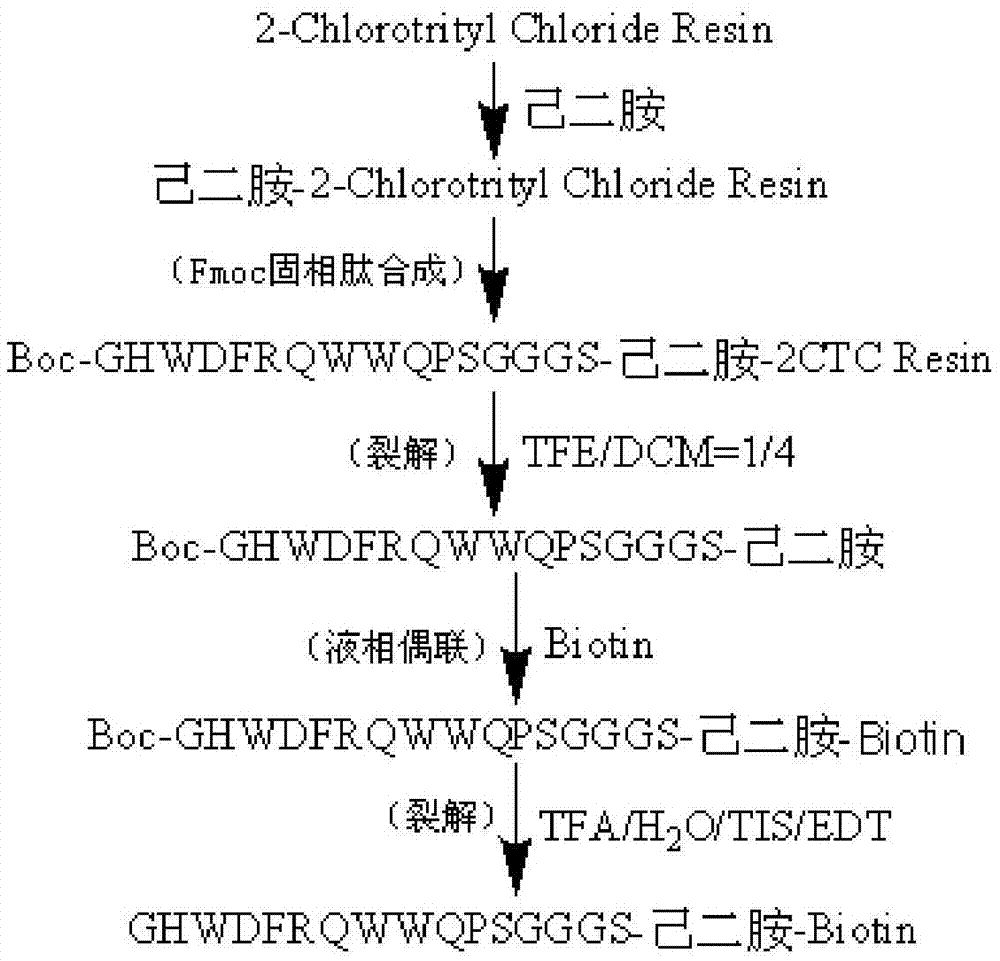
C-terminal modification peptide analysis method
A synthetic method and technology of modifying groups, which are applied in the field of synthesis of C-terminal modified peptides, can solve the problems of increasing difficulty in post-processing, increasing the difficulty of chemical synthesis of such peptides, and reducing the yield of target peptides.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1 NH 2 -(CH 2 ) 8 - Preparation of NH-2-Chlorotrityl Chloride Resin
[0024] Weigh 250 grams of 2-Chlorotrityl Chloride Resin with a degree of substitution of 0.8 mmol / g in a solid-phase reaction column, add DMF, and swell with nitrogen gas bubbles for 60 minutes; weigh 57.7 grams (400 mmol) of octyldiamine, dissolve it with DMF, and Add 90.0ml DIPEA (500mmol) in an ice-water bath at ℃, add to the reaction column, react for 1 hour, add 200ml methanol and 200ml DIPEA, mix and seal for 0.5h, wash with DCM three times, drain the resin after shrinking with methanol, and obtain NH 2 -(CH 2 ) 8 -NH-2-ChlorotritylChloride Resin.
Embodiment 2
[0025] Example 2 Fmoc-Phe-NH-(CH 2 ) 8 - Preparation of NH-2-Chlorotrityl Chloride Resin
[0026] Take by weighing the NH that embodiment 1 obtains 2 -(CH 2 ) 8 -NH-2-Chlorotrityl Chloride Resin 100 grams in the solid phase reaction column, add DMF, nitrogen bubble swelling for 60 minutes; weigh Fmoc-Phe-OH 23.22 grams (60mmol), HOBt9.72 grams (72mmol), HBTU 33.8 gram (60mmol), dissolved in DMF, 15.6ml DIPEA (72mmol) was added in an ice-water bath at 0°C, activated for 5 minutes, added to the reaction column, after 2 hours of reaction, added 70ml acetic anhydride and 60ml pyridine, mixed and sealed for 24 hours, washed with DCM three times , the resin is dried after methanol shrinkage, and Fmoc-Phe-NH-(CH 2 ) 8 -NH-2-ChlorotritylChloride Resin, 124g of resin with a detection substitution degree of 0.51mmol / g.
Embodiment 3
[0027] The preparation of embodiment 3 ibiratide peptide resin
[0028] The structure of ibiratide is: H-Met(O 2 )-Glu-His-Phe-D-Lys-Phe-NH-(CH 2 ) 8 -NH 2 .
[0029] Fmoc-Phe-NH-(CH 2 ) 8 - NH-2-ChlorotritylChloride Resin 98 g (50 mmol) was placed in a solid-phase reaction column, DMF was added, and nitrogen bubbled to swell for 60 minutes; the Fmoc protecting group was removed with DBLK, and washed 6 times with DMF. Weigh 468 g (100 mmol) of Fmoc-D-Lys(Boc)-OH, 16.3 g (120 mmol) of HOBt, dissolve in DMF, add 15.1 g of DIC (120 mmol) in an ice-water bath at 0°C, activate for 5 minutes, and add to the reaction column. After 2 hours of reaction, the resin was washed three times with DMF, the Fmoc protecting group was removed with DBLK, six times with DMF, and three times with DCM. Repeat the above coupling operation, and sequentially couple Fmoc-Phe-OH, Fmoc-His(Trt)-OH, Fmoc-Glu(OtBu)-OH, Boc-Met(O 2 )-OH. After the reaction was completed, it was shrunk with methano...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com