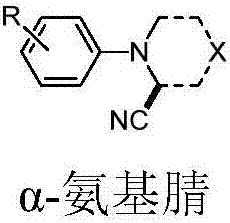
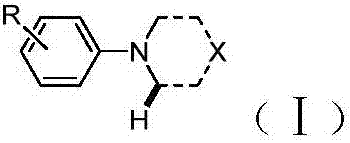
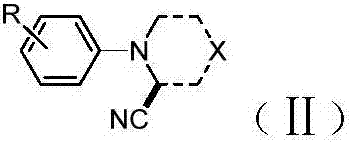
Method for synthesizing alpha-aminonitrile by adopting AIBN as individual cyan source
A technology of aminonitrile and cyano groups, applied in the field of α-aminonitrile compounds, can solve the problem that the nitrile source cannot get rid of the severe toxicity, and achieve the effect of high yield and simple method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-16
[0019] Synthesis method: 1.0 mol of tertiary amine 2 was added into a sealed tube, and then 3.0 mol of AIBN, 1.5 mol of pivalic acid, 1.0 mol of sodium acetate, molecular sieves, and methanol were added as solvents. The reaction was heated to 90°C for 8 hours, then cooled to room temperature, and separated by silica gel column chromatography. The flushing agent is petroleum ether / ethyl acetate (5:1 v / v). The results are shown in Table 1. The reaction formula is as follows:
[0020]
[0021] Table 1
[0022]
[0023]
[0024]
[0025]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com