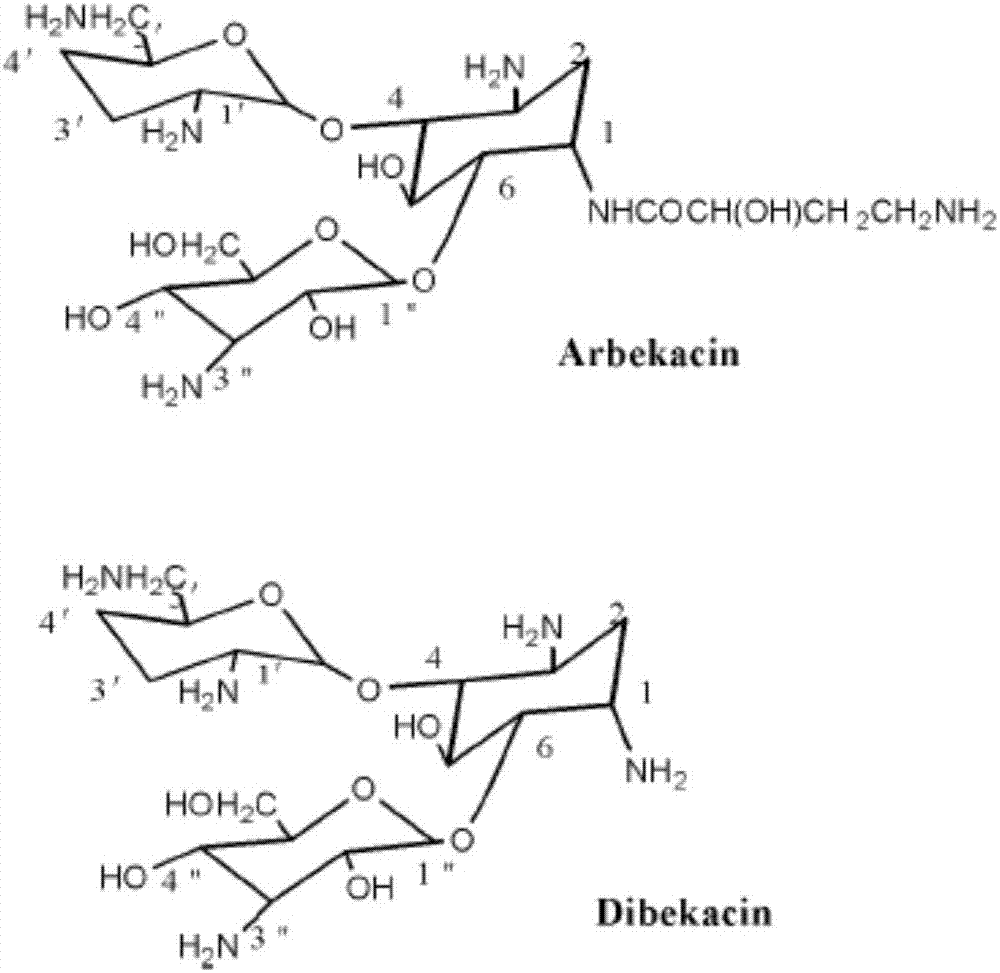
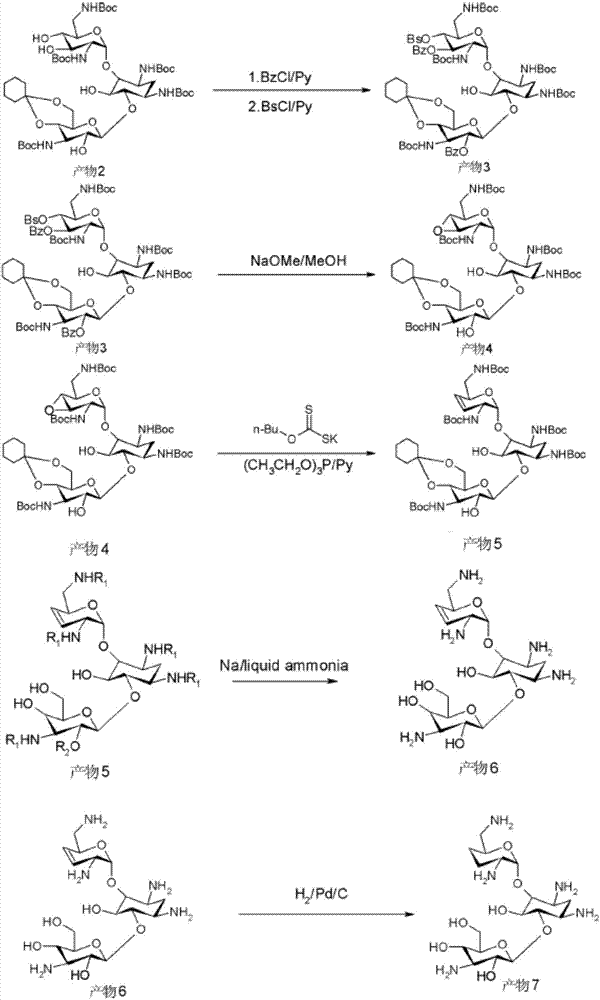
Preparation method for Arbekacin intermediate Dibekacin
A technology of rebekacin and arbekacin, which is applied in the field of preparation of arbekacin intermediate dibekacin, can solve the problems of complicated process and long synthesis route of dibekacin, and achieves simplified synthesis process, The effect of high yield and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] The preparation method of the arbekacin intermediate dibekacin of the present embodiment comprises the following steps:
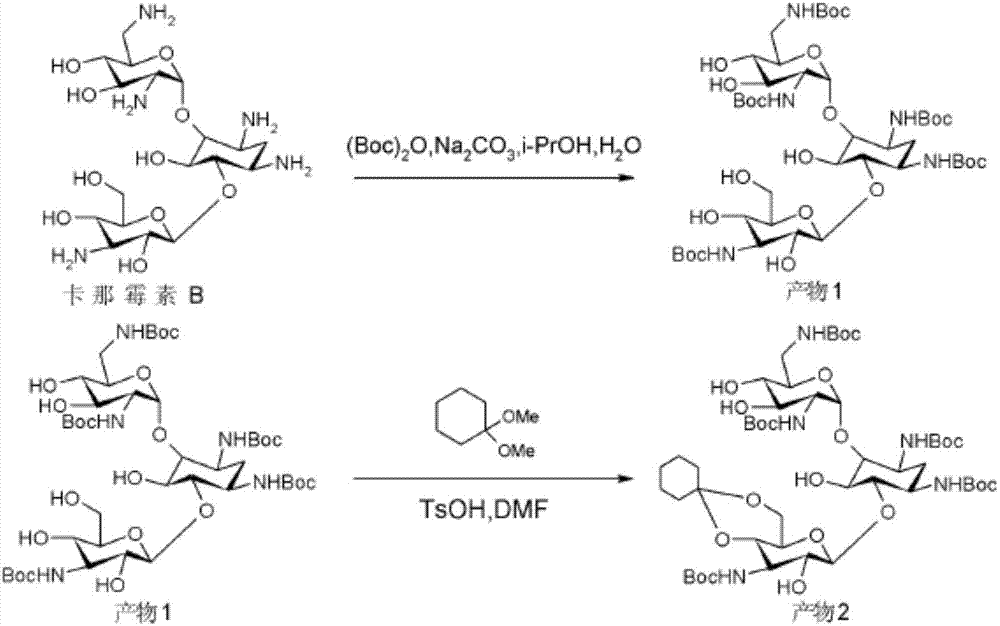
[0026] ① For the ring-forming protection of the hydroxyl group at the 4″ and 5″ positions of kanamycin B, the reaction formula involved is as follows.
[0027]
[0028] Firstly, 21.64g (114mmol, 5.5eq) of p-toluenesulfonic acid monohydrate was dehydrated under vacuum at 90°C, and after dehydration was completed, it was cooled to room temperature for use.
[0029] Dissolve the dehydrated p-toluenesulfonic acid in 100 mL of dimethylformamide (hereinafter referred to as DMF), and continue to add 10 g (20 mmol) of dry kanamycin B (code name KB) and 12.4 mL ( 82mmol, 4eq, eq=equivalent (molar equivalent) PhCH(OMe) 2 . The obtained reaction material was stirred at room temperature in a rotary evaporator equipped with double-pump vacuuming for 2.5h-4h (3h in this example), and the second vacuum pump could be turned off in case of severe evaporation. F...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com