Method for preparing nano-scale lithium manganese phosphate
A lithium manganese phosphate, nano-scale technology, applied in the direction of nanotechnology, nanotechnology, chemical instruments and methods, etc., can solve the problems of easy agglomeration, high energy consumption of solid phase method, uneven product particle size, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
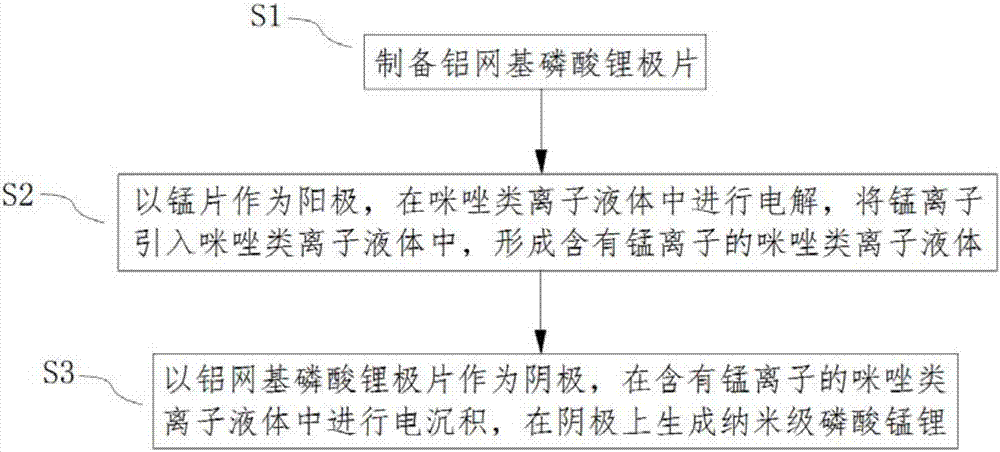
[0034] refer to figure 1 , In this embodiment, a method for preparing nanoscale lithium manganese phosphate is provided. The method comprises the steps of:
[0035] S1, preparing aluminum grid-based lithium phosphate pole pieces;
[0036] S1, using the manganese sheet as the anode, electrolyzing in the imidazole ionic liquid, introducing manganese ions into the imidazole ionic liquid to form the imidazole ionic liquid containing manganese ions;
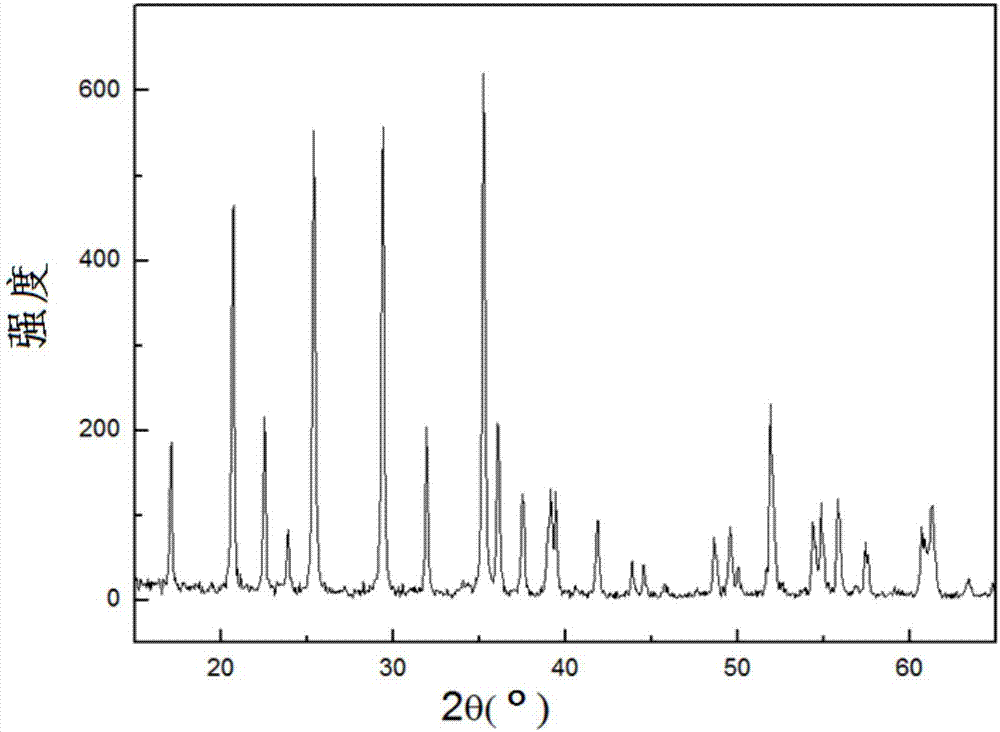
[0037] S3. Electrodeposition is performed in an imidazole ionic liquid containing manganese ions by using an aluminum mesh-based lithium phosphate pole piece as a cathode, and nanometer lithium manganese phosphate is generated on the cathode.
[0038] The imidazole-based ionic liquid containing manganese ions used in this example is an organic molten salt in a liquid state at or near room temperature. Compared with traditional organic solvents, it is completely composed of anions and cations, and the relatively high ionic environme...
Embodiment 2
[0056] In this embodiment, step S1 includes the following sub-steps:
[0057] S1.1, 100mL of LiOH·H with a concentration of 1mol / L 2 O solution was stirred and heated to 50°C and kept constant;
[0058] S1.2, using a peristaltic pump in LiOH·H 2 Add 50 mL of H with a concentration of 0.6 mol / L dropwise into the O solution 3 PO 4 Solution, the dropping rate is 5mL / min;
[0059] S1.3. Leave the solution formed in step S1.2 to stand, and the chemical reaction 3LiOH+H occurs 3 PO 4 → Li 3 PO 4 (S)+3H 2 O gives the precipitated product solid Li 3 PO 4 ;
[0060] S1.4. Wash the precipitated product, dry it in vacuum, and then calcinate at 300°C for 4h to form white Li 3 PO 4 Powder;
[0061] S1.5, the aluminum mesh and the white Li 3 PO 4 The powder is pressed into an aluminum mesh-based lithium phosphate pole piece by a tablet machine, wherein, during the pressing process, the pressure is 10 MPa, and the pressure is maintained for 2 minutes. The aluminum mesh base ...
Embodiment 3
[0071] Specifically, step S1 includes the following sub-steps:
[0072] S1.1, 100mL of LiOH·H with a concentration of 1mol / L 2 O solution was stirred and heated to 50°C and kept constant;
[0073] S1.2, using a peristaltic pump in LiOH·H 2 Add 50 mL of H with a concentration of 0.6 mol / L dropwise into the O solution 3 PO 4 Solution, the dropping rate is 5mL / min;
[0074] S1.3. Leave the solution formed in step S1.2 to stand, and the chemical reaction 3LiOH+H occurs 3 PO 4 → Li 3 PO 4 (S)+3H 2 O, to obtain the precipitated product solid Li 3 PO 4 ;
[0075] S1.4. Wash the precipitated product, dry it in vacuum, and then calcinate at 300°C for 4h to form white Li 3 PO 4 Powder;
[0076] S1.5, the aluminum mesh and the white Li 3 PO 4 The powder is pressed into an aluminum mesh-based lithium phosphate pole piece by a tablet machine, wherein, during the pressing process, the pressure is 10 MPa, and the pressure is maintained for 2 minutes. The aluminum grid base l...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The inside diameter of | aaaaa | aaaaa |
| Wall thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com