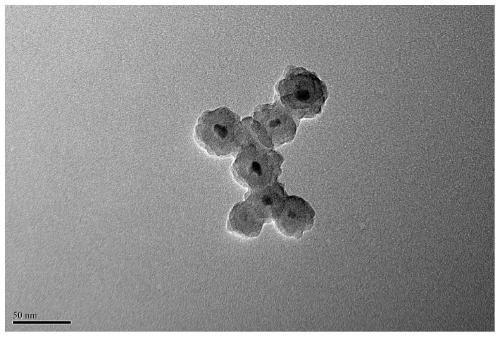
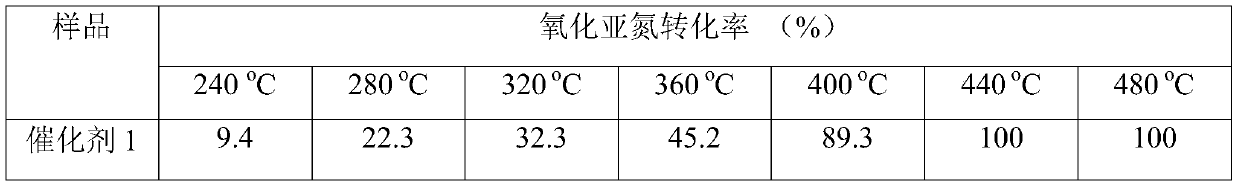
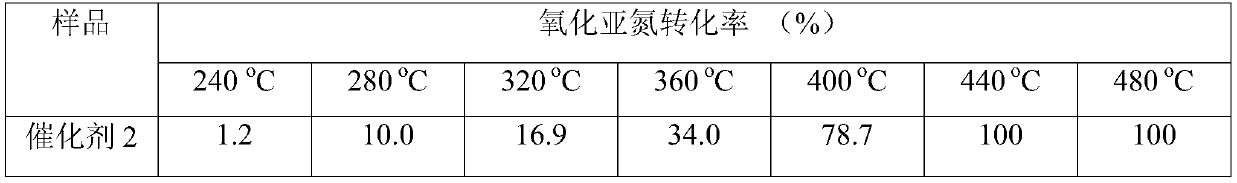
A medium and low temperature catalytic decomposition n 2 Catalyst and preparation method and application of o
A low-temperature catalysis and catalyst technology, applied in the direction of metal/metal oxide/metal hydroxide catalyst, physical/chemical process catalyst, separation method, etc. Active oxide sintering and other issues to achieve superior low-temperature catalytic activity, maintain continuous adsorption and desorption, and stable configuration characteristics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] (a) Weigh 1.82g Co(NO 3 ) 2 ·6H 2 O, 1.61g Co(acac) 2 (The total amount of Co ions is 0.0125mol, and the mole fraction ratio of inorganic salts and organic salts is 1:1) and 35.1g (0.25mol) of hexamethylenetetramine are added to 1L of ethanol aqueous solution with a volume ratio of 2:1 and stirred Mix and add 3.43 g of polyvinylpyrrolidone to the mixed solution until complete dissolution. (b) Continue to add 15 mL of 3wt% H to the mixed solution 2 o 2 solution, after the solution system gradually turned black, the temperature was raised to 60° C., and vigorously stirred for 72 hours to obtain highly uniformly dispersed cobalt trioxide nanoparticle suspension. (c) 0.014g Zr(NO 3 ) 2 ·5H 2 O was dissolved in 210 mL of ethanol, and 30 mL of ethyl orthosilicate was added thereto to obtain a mixed solution of ethyl orthosilicate, zirconium nitrate and ethanol. (d) Add the mixed solution prepared in step (c) dropwise in situ to the suspension prepared in step (b), an...
Embodiment 2
[0046] (a) Weigh 1.19g CoCl 2 ·6H 2 O, 2.49g Co(Ac) 2 4H 2 O (the total amount of Co ions is 0.015mol, and the mole fraction ratio of its inorganic salts and organic salts is 0.5:1) and 42.2g (0.3mol) of hexamethylenetetramine are added to 1L of ethanol aqueous solution with a volume ratio of 2.5:1 Stir to mix, and add 3.66 g of polyvinylpyrrolidone to the mixed solution until complete dissolution. (b) Continue to add 20mL of 3wt%H to the mixed solution 2 o 2 solution, after the solution system gradually turned black, the temperature was raised to 55° C., and vigorously stirred for 12 hours to obtain a highly uniformly dispersed suspension of cobalt trioxide nanoparticles. (c) 0.32g Zr(NO 3 ) 2 ·5H 2 O was dissolved in 150 mL of ethanol, and 15 mL of ethyl orthosilicate was added thereto to obtain a mixed solution of ethyl orthosilicate, zirconium nitrate and ethanol. (d) Add the mixed solution prepared in step (c) dropwise in situ to the suspension prepared in step (...
Embodiment 3
[0051] (a) Weigh 1.82g Co(NO 3 ) 2 ·6H 2 O, 1.61g Co(acac) 2 (The total amount of Co ions is 0.0125mol, and the mole fraction ratio of inorganic salts and organic salts is 1:1) and 28.1g (0.2mol) of hexamethylenetetramine are added to 1L of ethanol aqueous solution with a volume ratio of 2:1 and stirred Mix and add 3.43 g of polyvinylpyrrolidone to the mixed solution until complete dissolution. (b) Continue to add 18 mL of 3 wt% H to the mixed solution 2 o 2 solution, after the solution system gradually turned black, the temperature was raised to 70° C., and vigorously stirred for 24 hours to obtain a highly uniformly dispersed suspension of cobalt trioxide nanoparticles. (c) 0.25g Zr(NO 3 ) 2 ·5H 2 O was dissolved in 210 mL of ethanol, and 30 mL of ethyl orthosilicate was added thereto to obtain a mixed solution of ethyl orthosilicate, zirconium nitrate and ethanol. (d) Add the mixed solution prepared in step (c) dropwise in situ to the suspension prepared in step (b...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com