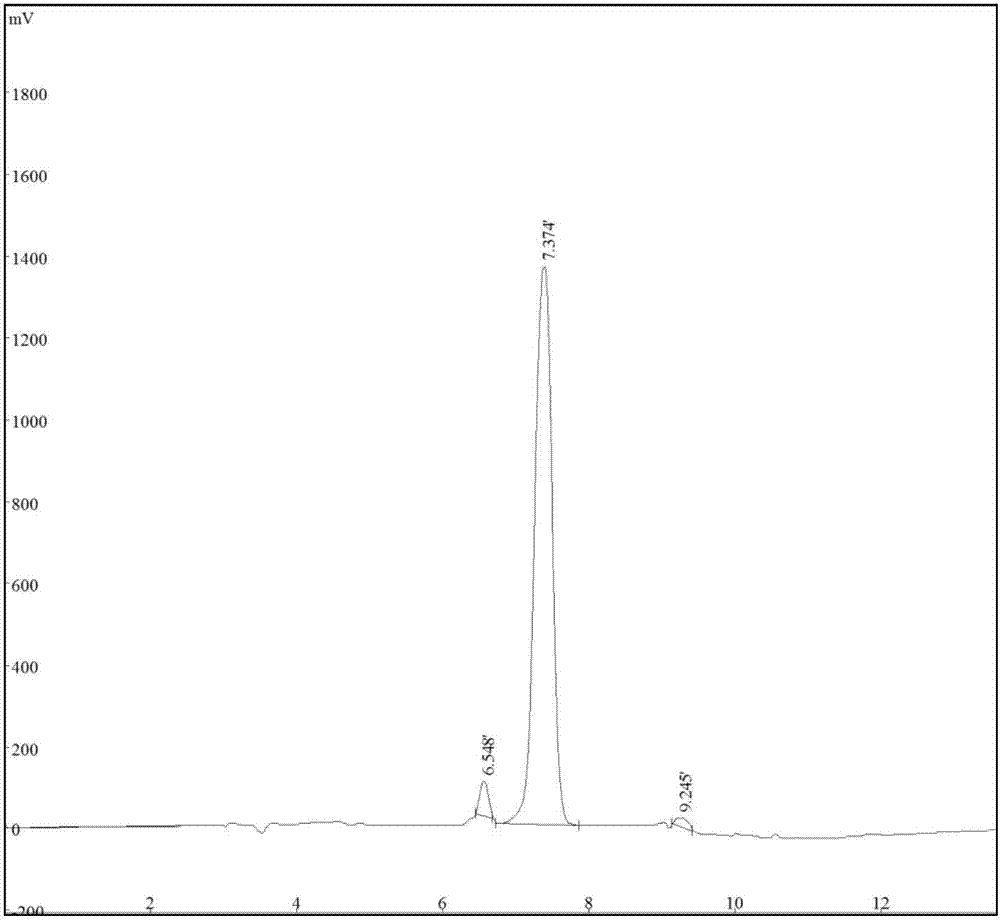
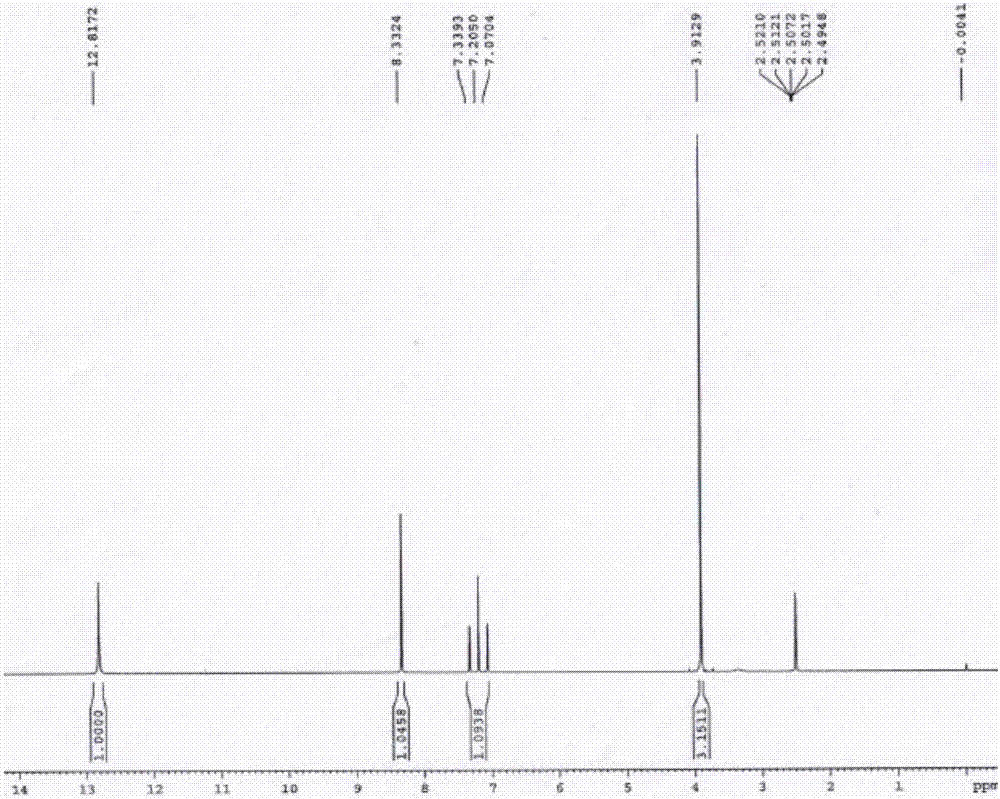
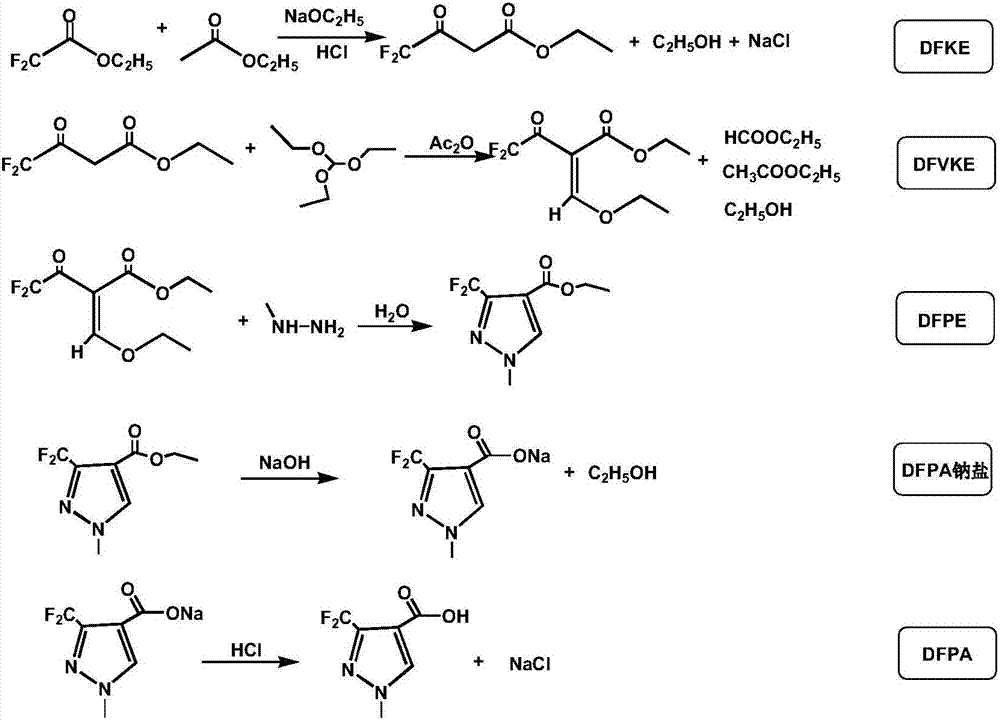
3-difluromethyl-1-methyl-pyrazole-4-formic acid and synthetic method thereof
A technology of difluoromethyl group and synthesis method, applied in the field of 3-difluoromethyl-1-methyl-pyrazole-4-carboxylic acid and its synthesis, can solve the problem of low yield, rare raw materials, potential safety hazards, etc. problem, to achieve the effect of simple reaction conditions, high purity and few impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] a. Synthesis of DFKE: At a temperature of 0°C, add sodium ethoxide to ethyl acetate and stir the reaction, then add ethyl difluoroacetate, the molar ratio of ethyl acetate, organic base and ethyl difluoroacetate is 2:1: 1. Stir the reaction at a temperature of 3°C for 15 minutes, then raise the temperature to 20°C and keep it warm for 3.5 hours; after heat preservation, cool down to 1°C and pass in hydrogen chloride until the pH value reaches 2, and add dichloromethane to the solution, concentrate under reduced pressure to obtain concentrated liquid 1 and filter cake; filter cake was added dichloromethane beating 2 times, combined 2 times beating filtrate, concentrated under reduced pressure to obtain concentrated liquid 2, mixed concentrated liquid 1 and concentrated liquid 2 to obtain DFKE;
[0024] b. Synthesis of DFVKE: Add acetic anhydride to the DFKE obtained in step a, add triethyl orthoformate dropwise at 95°C and keep it warm for 1h; then cool down to 60°C, conc...
Embodiment 2
[0028] a. Synthesis of DFKE: Add a mixture of potassium ethylate and pyridine to ethyl acetate at a temperature of 5°C, add ethyl difluoroacetate after stirring and reacting, the molar ratio of ethyl acetate, organic base and ethyl difluoroacetate 3:1.1:1, stirred and reacted at a temperature of 10°C for 20min, then raised to 20°C and kept warm for 4h; after heat preservation, cooled to 0°C and introduced hydrogen chloride until the pH value reached 3, and added dichloromethane to the solution, and reduced Concentrate under pressure to obtain concentrated solution 1 and filter cake; add dichloromethane to the filter cake for beating 3 times, combine the beating filtrate for 3 times, concentrate under reduced pressure to obtain concentrated solution 2, and mix concentrated solution 1 and concentrated solution 2 to obtain DFKE;
[0029] b. Synthesis of DFVKE: Add acetic anhydride to the DFKE obtained in step a, add triethyl orthoformate dropwise at 100°C and keep it warm for 0.8h...
Embodiment 3
[0033] a. Synthesis of DFKE: Add a mixture of triethylamine and diisopropylethylhexylamine to ethyl acetate at a temperature of 5°C, add ethyl difluoroacetate, ethyl acetate, organic base and The molar ratio of ethyl difluoroacetate is 2.3:1:1. Stir the reaction at a temperature of 6°C for 15 minutes, then raise the temperature to 23°C and keep it warm for 4 hours; Add dichloromethane to the solution, concentrate under reduced pressure to obtain concentrated solution 1 and filter cake; add dichloromethane to the filter cake for beating twice, combine the filtrate from two times of beating, concentrate under reduced pressure to obtain concentrated solution 2, and mix concentrated solution 1 and concentrated solution 2 get DFKE;
[0034] b. Synthesis of DFVKE: Add acetic anhydride to the DFKE obtained in step a, add triethyl orthoformate dropwise at 100°C and keep it warm for 1h; then cool down to 40°C, concentrate under reduced pressure to a temperature of 110°C, and vacuum deg...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com