Application of triptolide and triptolide derivative to preparation of medicine for treating and/or preventing lung injury diseases
A technology for triptolide and lung injury, which is applied to the application field of triptolide and its derivatives in the preparation of medicines for treating and/or preventing lung injury diseases, and can solve the problem that triptolide and triptolide have not yet been seen. Problems such as terpenoids
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
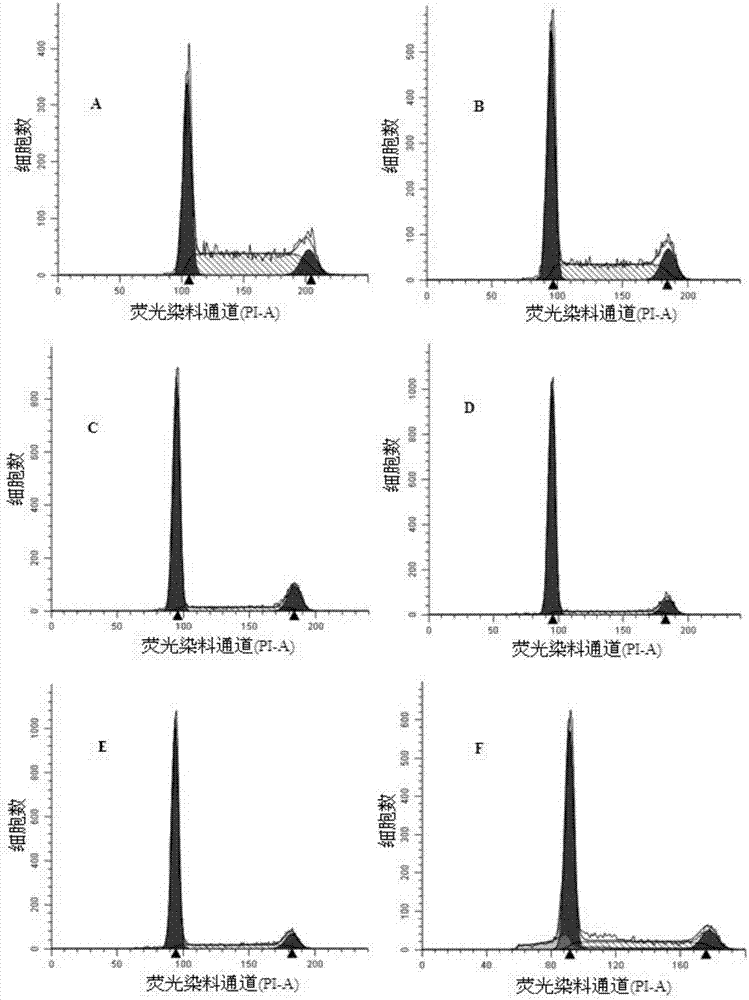
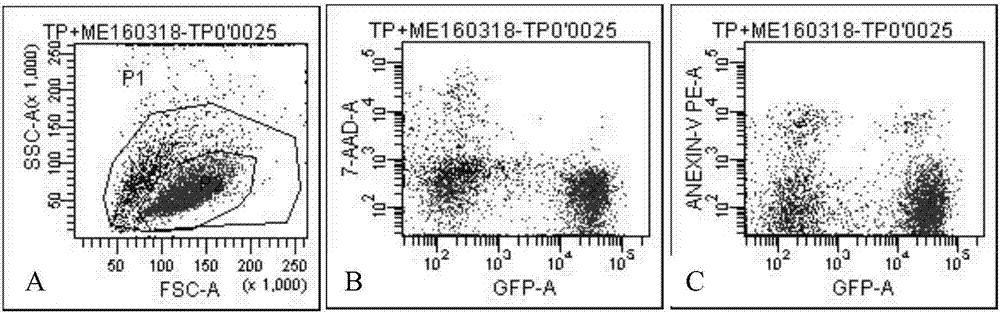
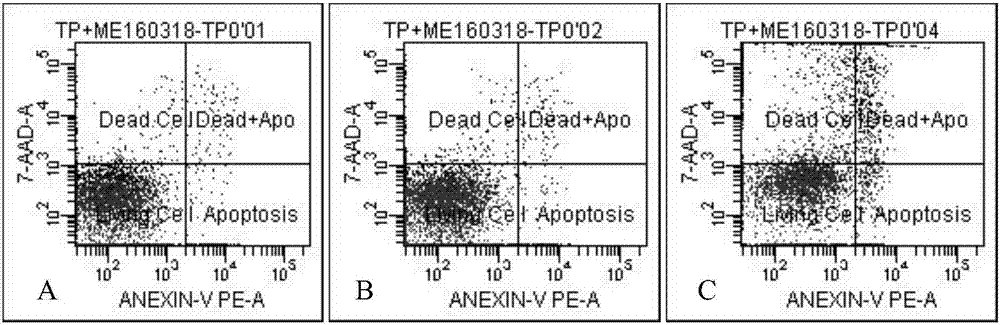
Image
Examples
Embodiment 1
[0125] Preparation of Triptolide Oral Tablets: Triptolide Oral Tablets use β-cyclodextrin as an auxiliary material, which can reduce the stimulating effect of Triptolide on the gastrointestinal tract. The specific preparation method is: firstly, triptolide Dissolve the powder in ethanol to form a 100μM (36000μg / L) solution, mix β-cyclodextrin and triptolide at a mass ratio of 10:1-1000:1, stir thoroughly and then treat with ultrasonic waves (ultrasonic frequency 40000Hz) for 10 -30 minutes to prepare the triptolide β-cyclodextrin inclusion compound, dry it and make it into a tablet coated with sugar coating to make a finished medicine. Each tablet contains 40 μg of triptolide, which is divided into three doses at a dose of 0.5-10 μg / kg / day.
Embodiment 2
[0127] Inhalants through the respiratory tract (through the oral intake tube) inhalation route: triptolide is insoluble in water, in order to improve water solubility, try to disperse triptolide molecules, first dissolve triptolide powder in DMSO to 100μM (36mg / L) solution, add chitosan by weight / volume ratio and make concentration 0.6% (w / v) solution, after this solution is filtered through 0.8 μm microporous membrane, spray drier prepares microparticle, parameter setting is as follows: Nozzle diameter 0.5mm, inlet temperature 110°C, air velocity 0.7m 3 min -1 , atomization pressure 200kpa. When using nebulization or powder spray, daily inhalation of 0.01-2ug / kg body weight / day triptolide equivalent medicine, three times a day.
Embodiment 3
[0129] Oral solution: first dissolve triptolide powder in ethanol to form a 100μM (36000μg / L) solution, heat and mix until completely dissolved, slowly add 5 times the volume of water, keep the water temperature at 80°C, add appropriate amount of cyclamate and antiseptic After mixing, let it cool and stand for 24 hours, then remove the ethanol by suction filtration until the concentration is less than 0.5% (v / v). During this process, gradually add warm water at 80°C to supplement the lost volume, and pack it into 10ml graduated glass bottles , each bottle contains triptolide 60ug, 0.22um membrane filter sterilization, sterile seal. Take it three times according to the amount of 0.5-10μg / kg / day.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com