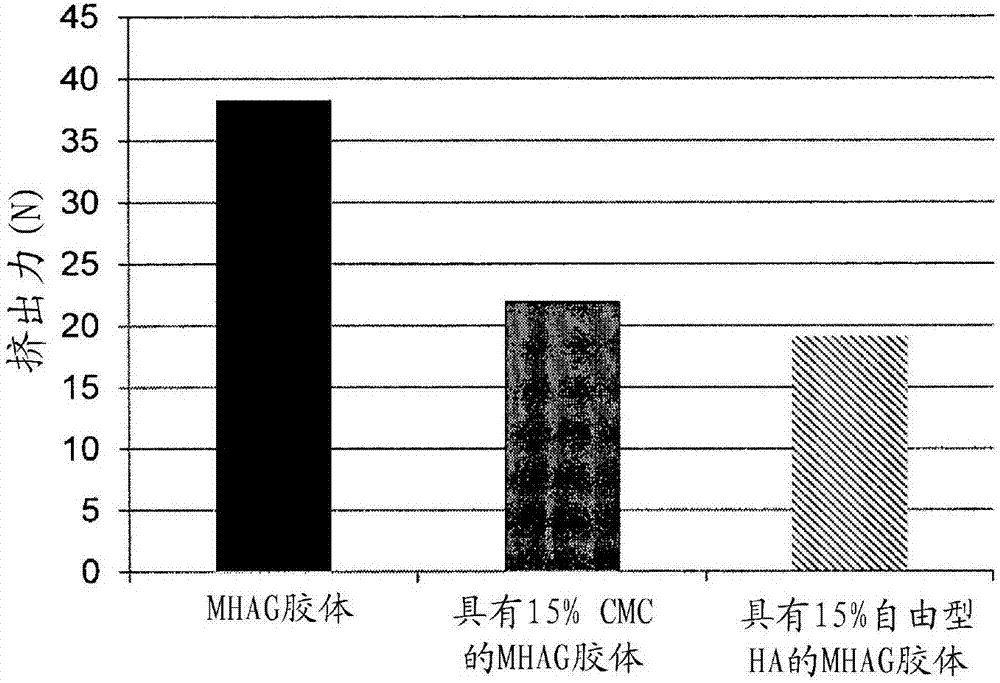
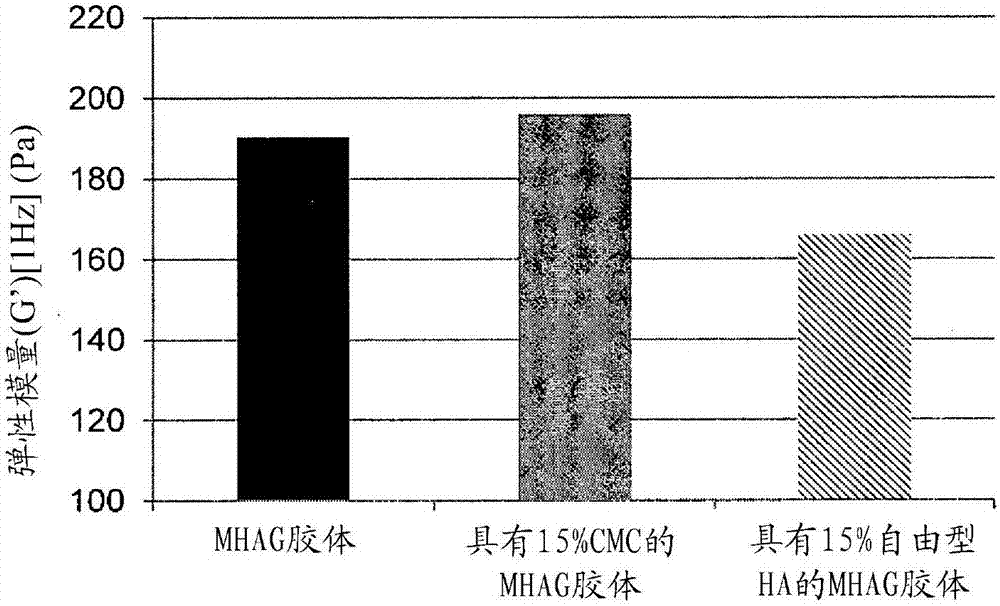
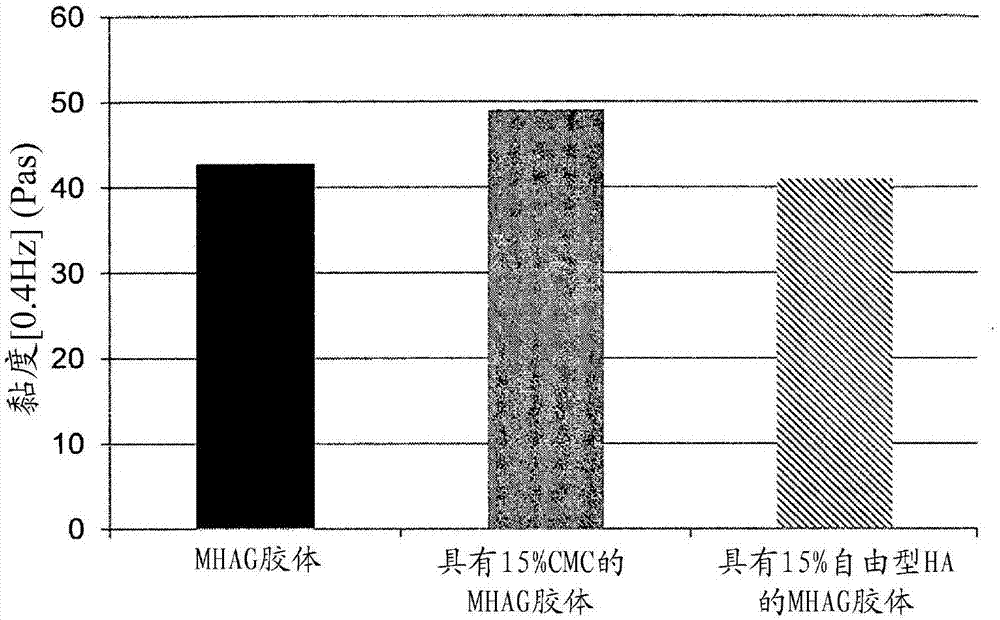
Dermal filler based on crosslinked hyaluronic acid and carboxymethyl cellulose lubricant
一种羧甲基纤维素、填充剂的技术,应用在麻醉剂、泌尿系统疾病、医药科学等方向,能够解决填充效果降低等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0094] Preparation of HA colloids (MHAG colloids and MHAI colloids) without lubricating phase and with or without CaHAP particles (compare colloid)
[0095] Preparation of crosslinking solution
[0096] The HA "cake" was prepared by dissolving 43 g of sodium hyaluronate (average molecular weight about 2.8 MDa) in 270.35 g of phosphate buffer. The resulting HA blocks can be stored in the refrigerator until needed. Alternatively, an alkaline solution was prepared by dissolving 3.31 g of solid sodium hydroxide in 10 ml of buffer. In addition, a BDDE solution was prepared by mixing 12.5 g of 2M sodium hydroxide solution with 88.5 g of phosphate buffer, and then mixing 8.21 ml of the resulting solution with 3.395 ml of BDDE.
[0097] cross-linking
[0098] The HA mass was manually broken into small pieces and all of the alkaline solution was added to the cup followed by mixing at 12 rpm for 30 to 40 minutes. Next, the BDDE solution was added to the cup and mixing was co...
Embodiment 2
[0106] Preparation of HA colloids with 15% CMC as lubricating phase (colloids of the invention)
[0107] Solution "LB1" was prepared by adding 62.75 g of glycerol to 2.150 g of lidocaine hydrofluoric acid and dissolving the mixture in 135.142 g of phosphate buffer. Next, stir gently using a magnetic stirrer until complete dissolution.
[0108] Next, 2.764 g of sodium carboxymethylcellulose (NaCMC) was vigorously mixed with 105.24 g of LB1 in a cup for 1 hour. After degassing, 392.025 g of the MHAG colloid prepared in Example 1 was added and mixed moderately for 1.5 hours. After an additional degassing step, 1 ml syringes were filled and sterilized at 127°C for 4 minutes.
Embodiment 3
[0110] Preparation of HA colloids with 15% (v / v) free HA as lubricating phase (comparative colloids)
[0111] Solution "LB2" was prepared by dissolving 1.131 g of lidocaine hydrochloride in 72.743 g of phosphate buffer. Next, 1.170 g of sodium hyaluronate (2.5-3.0 MDa) was added. After complete dissolution, 33.005 g of glycerol was added. The mixture was then stirred at a moderate speed for 1 hour and 30 minutes and maintained at 5°C until used.
[0112] HA gel with 15% (v / v) free HA lubricant was prepared by mixing 106.721 g of LB2 with 387.357 g of the MHAG gel prepared in Example 1. Moderate mixing was maintained for 2 hours. After degassing, the mixture was transferred to a 1 ml syringe and sterilized at 127°C for 4 minutes.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com