Preparation method of beta-configuration decitabine precursor
A decitabine and configuration technology, applied in the field of preparation of decitabine precursor, can solve the problem of unfavorable enrichment of β-type products, and achieve the effect of simple post-processing, good repeatability and stable purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] 60mmol of 2,4-bis-(trimethylsilyl)-5-azacytosine and 50mmol of 1-(2-butenyl)oxy-2-deoxy-3,5-di-O-acetyl -D-Ribofuranose was dissolved in 500 mL of acetonitrile, the temperature was controlled to 0°C, 10 mmol of trifluoromethanesulfonic acid was added, and kept at 0°C, the reaction was stirred for 2 hours, and the reaction was completed by HPLC monitoring. Quickly quench the reaction and wash the reaction mixture with 500 mL saturated aqueous sodium bicarbonate solution, separate the organic layer, evaporate it to dryness under vacuum, and then recrystallize with toluene to obtain 14.75 g of solid, which is identified as 1-(2- Deoxy-3,5-di-O-acetyl-D-ribose)-4-amino-1,3,5-s-triazin-2-one, its purity is 90% by HPLC, wherein the β configuration and α The content ratio of the configuration is 2.5.
[0019] 1 H-NMR (DMSO-d6) δ: 2.0(s,6H), 2.2-3.0(m,2H), 4.1(m,1H), 4.3(m,1H), 4.8(m,1H), 5.2(m ,1H),6.1(m,1H),7.5(s,1H),7.6(s,1H),8.4(s,1H)
Embodiment 2-4
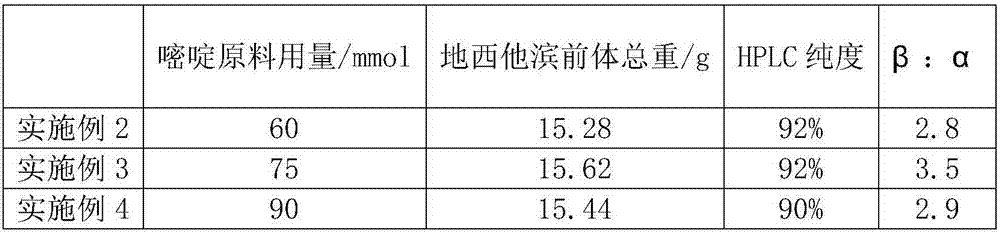
[0021] Use 1-allyloxy-2-deoxy-3,5-di-O-acetyl-D-ribofuranose to directly carry out the coupling reaction, and each condition refers to the example cytosine raw material 1,2,4-di-( The amount of trimethylsilyl)-5-azacytosine is different, and the results are summarized as follows:
[0022]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com