Preparation method of imidocarb
A technology of imidazolium and imidazoline, which is applied in the field of preparation of pharmaceutical chemicals, can solve the problems of long production cycle, difficult material-liquid separation, slow rate, etc., and achieve the advantages of simple post-treatment operation, overcoming cumbersome operation, and easy reaction process Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
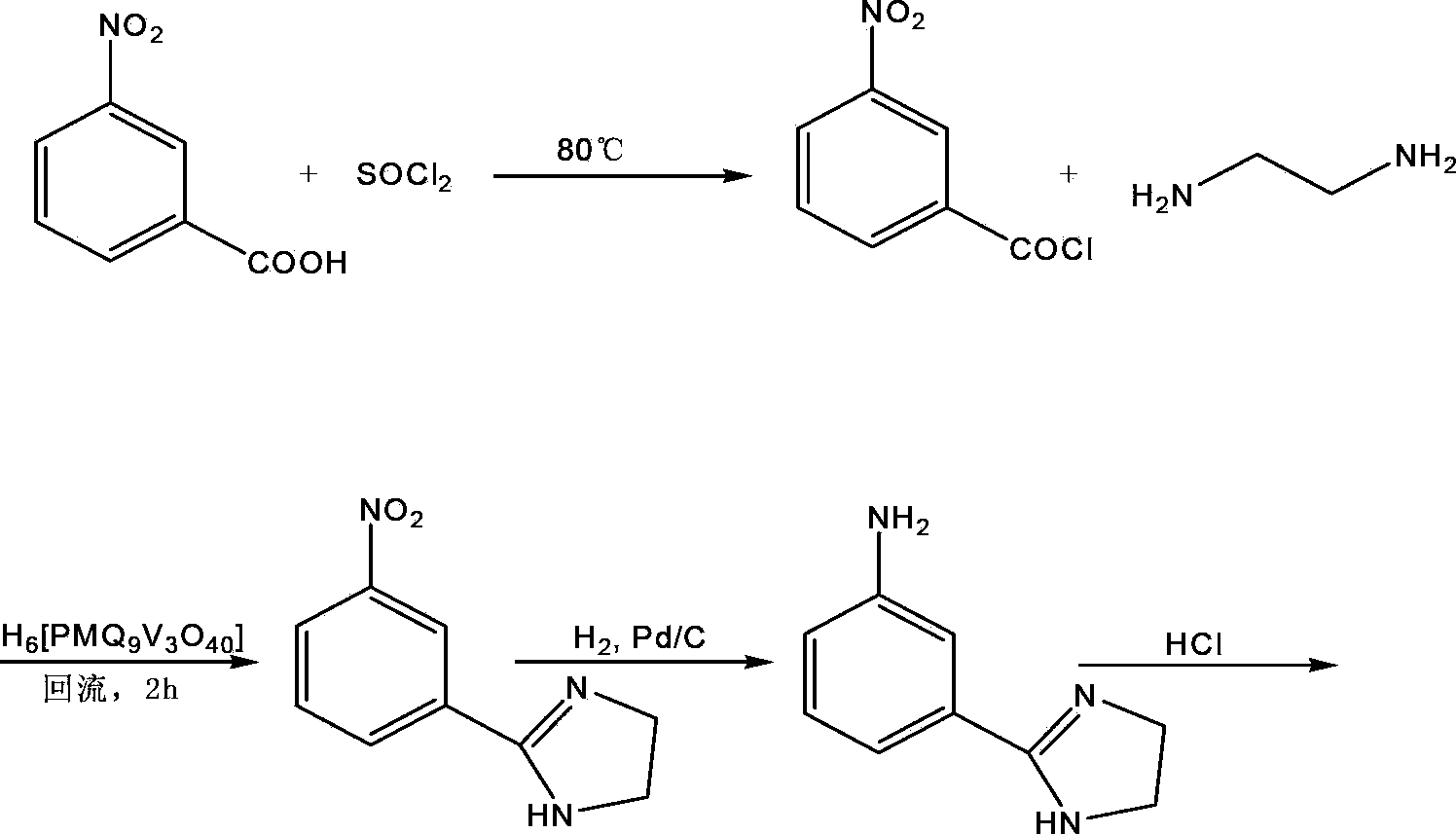
[0043] A preparation method of imidazolide, the steps are as follows:
[0044] (1) Add 121.0kg of m-nitrobenzoic acid and 344.9kg of thionyl chloride to the reaction kettle, mix and stir, heat up to 80°C, reflux for 4h, control the temperature at 75°C and concentrate under reduced pressure to remove the thionyl chloride. Prepare at room temperature Obtain 134.1 kg of m-nitrobenzoyl chloride, which is a yellow solid of m-nitrobenzoyl chloride with a molar yield of 99.8%;
[0045] (2) Add 133.3kg of m-nitrobenzoyl chloride prepared in step (1) to 600L acetonitrile, then add 8.0kg of phosphomolybdovanadium heteropoly acid, stir and add 47.3kg of ethylenediamine, stir and heat to 80°C, reflux The reaction was carried out for 2 hours, filtered, and the filtrate was taken and concentrated under reduced pressure at 75°C to obtain 126.5 kg of yellow-green 2-(3-nitrophenyl) imidazoline solid with a molar yield of 91.6%.
[0046] (3) Add 124.4 kg of 2-(3-nitrophenyl) imidazoline prepared in s...
Embodiment 2
[0050] The preparation method as described in Example 1, except that:
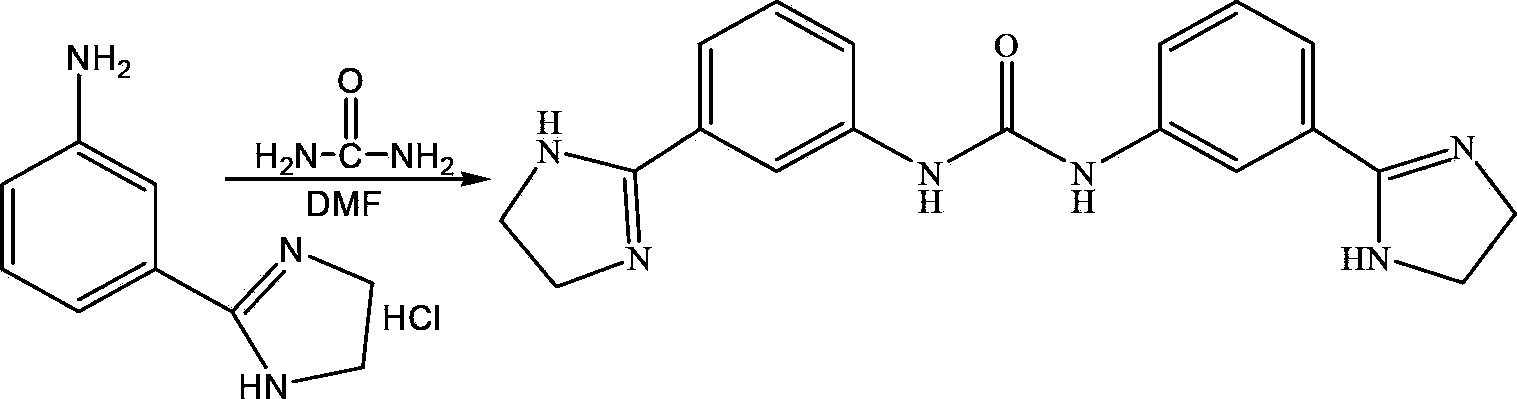
[0051] (4) Add 122.4kg of the prepared 2-(3-aminophenyl)imidazoline hydrochloride to 700LN,N-methylformamide, stir and add 33kg of urea, stir and heat up to 155℃, react for 4.5h, and cool down To 0°C, stand to crystallize for 1h, filter and wash, and vacuum dry at 60°C to obtain 91.5kg of imidazolide white solid.
[0052] After testing, the prepared imidazocarb mp (melting point) is 350-352°C, the content is 98.9%, and the molar yield is 83.8%.
Embodiment 3
[0054] The preparation method as described in Example 1, except that:
[0055] (4) Add 120.5kg of the prepared 2-(3-aminophenyl)imidazoline hydrochloride to 700LN,N-dimethylformamide, stir and add 33kg of urea, stir and warm up to 160℃, react for 5h, and cool down To 3°C, stand for crystallization for 1h, filter and wash, and vacuum dry at 60°C to obtain 89.7kg of imidazolide white solid.
[0056] After testing, the prepared imidazocarb mp (melting point) is 350-352°C, the content is 99.1%, and the molar yield is 83.7%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com