DC (Dendritic Cell) vaccine modified by DUOX2 and applications of DC vaccine in killing and wounding pancreatic cancer initiating cells in targeted manner
A technology for initiating cells and pancreatic cancer, applied in the field of tumor cell immunotherapy, can solve problems such as failure to produce long-term therapeutic effects, failure to achieve therapeutic effects, and inability to effectively kill pancreatic cancer initiating cells.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
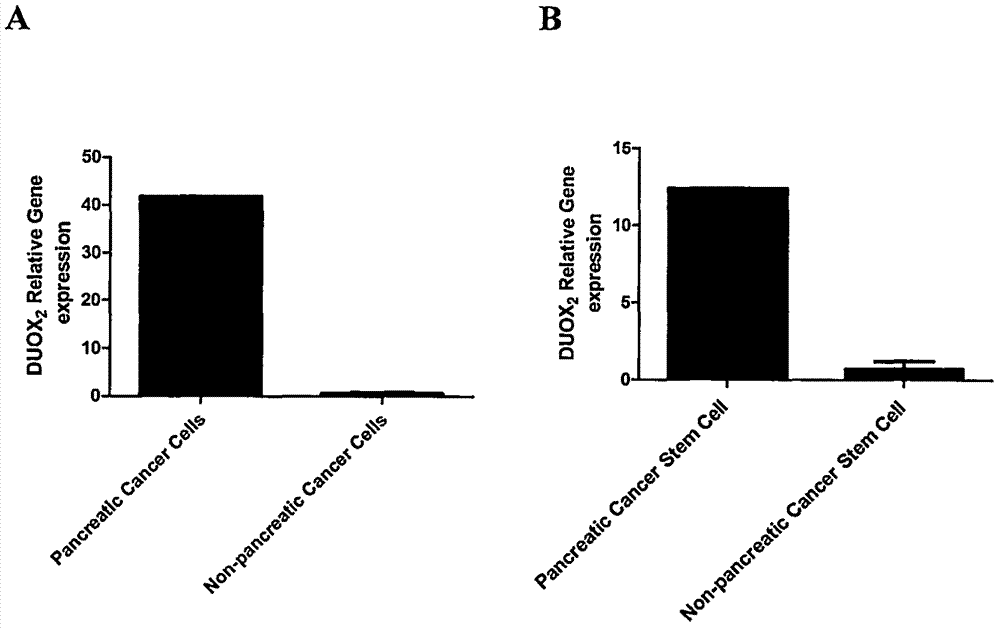
[0016] Example 1: Gene screening and identification of pancreatic cancer-initiating cells and pancreatic cancer-associated antigens
[0017] CD44 + CD24 + ESA + Pancreatic cancer-initiating cells and CD44 - CD24 - ESA - Gene profiles differentially expressed in non-pancreatic cancer-initiating cells and in pancreatic cancer and normal pancreatic tissue. Firstly, pancreatic cancer-initiating cells and non-pancreatic cancer-initiating cells were sorted out by flow cytometry, and the total RNA of pancreatic cancer-initiating cells and non-pancreatic cancer-initiating cells, as well as pancreatic cancer and normal pancreatic tissues were extracted respectively, and passed through Affymetrix Human Gene 1.0ST human gene expression profile chip scanning analysis, CD44 was initially screened out + CD24 + ESA + Pancreatic cancer-initiating cells and highly expressed gene DUOX in pancreatic cancer 2 , Real-time fluorescent quantitative polymerase chain reaction verified the scr...
Embodiment 2
[0018] Example 2: DUOX 2 Modified DC vaccine preparation
[0019] 1. Peripheral blood mononuclear cell isolation:
[0020] Take out the blood, slowly add it to the upper layer of Ficoll separation medium, centrifuge at 1000G for 30min, absorb the white PBMC at the boundary, and resuspend in 1640 medium; centrifuge at 350G for 8min, remove the supernatant, repeat three times, add 10ml of medium to resuspend and count. According to the counting results, DCs were cultured.
[0021] 2. Culture and transfection of DC
[0022] Adjust cell concentration to 3 x 10 6 / mL, take 6ml and add it to a T25 culture flask, at 37°C, 5% CO 2 Take it out after culturing in the incubator for 2 hours, discard the suspended cells, wash gently, add 6 mL containing GM-CSF (50ng / mL), IL-4 (50ng / mL), double antibody (100U / mL) and 10% FBS After 5 days, add 3mL containing IL-1β(10ng / mL), IL-6(100ng / mL), TNF-α(10ng / mL), PGE2(1μg / mL), CD40L with enhancer( 1 μg / mL), IFN-α (3000 μ / mL), IL-4 (800 U / mL), ...
Embodiment 3
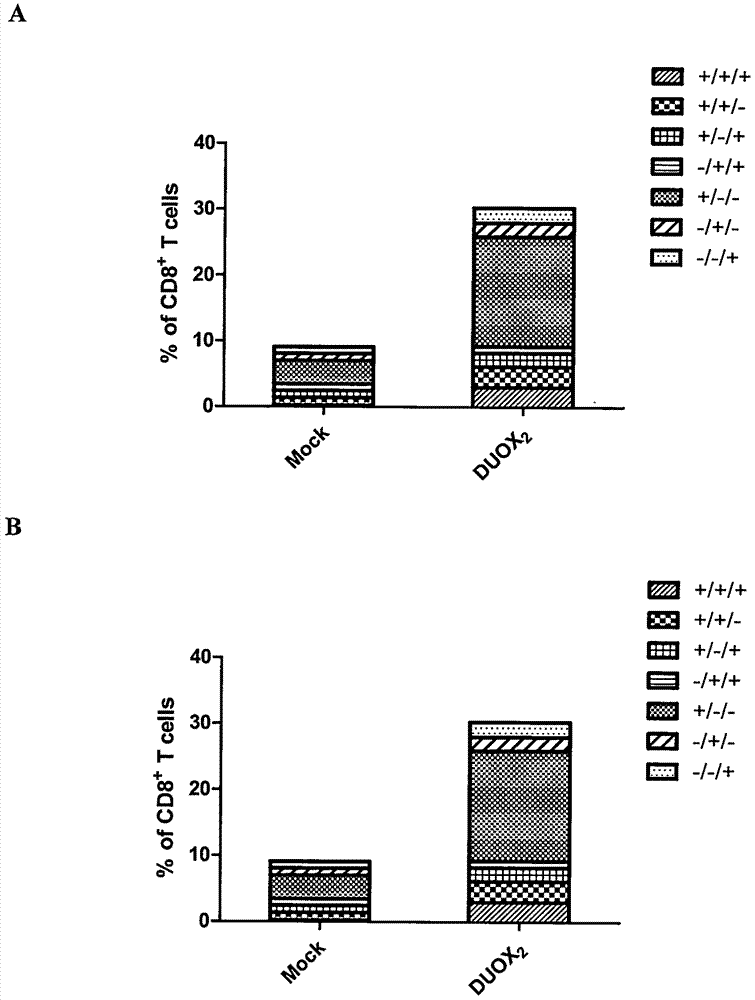
[0023] Example 3: DUOX 2 Modified DC vaccines induce specific T cell responses
[0024] 1. Antigen-specific T cell preparation and immunogenicity detection
[0025] will load DUOX 2 Antigen mature DC cells and T cells were mixed at a ratio of 1:10, added to lymphocyte culture medium containing final concentrations of 5ng / ml IL-7 and 20IU / ml IL-2 for culture, and the medium was changed every day, and obtained on the 7th day Load DUOX 2 Antigen DC cells stimulate T cells.
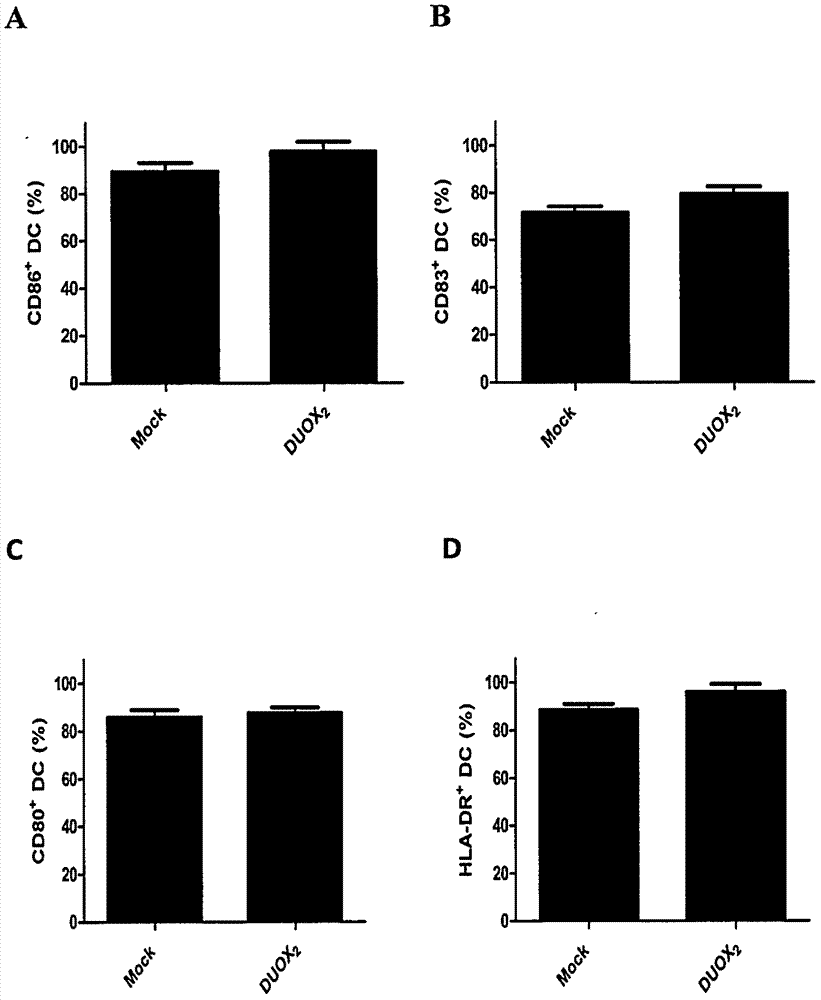
[0026] 2. Immunophenotype detection of mature DC cells
[0027] Take the mature DC cells, wash them twice with PBS, and take 1×10 5 / mL were added to the corresponding flow tubes. Add 1 μl of the monoclonal antibodies to be detected, including HLA-DR, CD80, CD83 and CD86 antibodies, and incubate at 4°C in the dark for 30 minutes. Washed twice with PBS, resuspended with 400 μl of PBS, and detected by flow cytometer Cytoflex (Beckman).
[0028] 3. Detection of immunogenicity of mature DC vaccine
[002...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com